 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.007.885 |
| Chemical and physical data | |
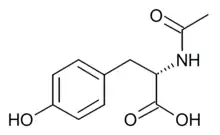
| Formula | C11H13NO4 |
| Molar mass | 223.228 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
N-Acetyl-L-tyrosine is an amino acid, an N-acetyl derivative of tyrosine. It is used for parenteral nutrition and as a dietary supplement.[1][2][3]
See also
References
- ↑ Hoffer LJ, Sher K, Saboohi F, Bernier P, MacNamara EM, Rinzler D (November 2003). "N-Acetyl-L-tyrosine as a tyrosine source in adult parenteral nutrition". Journal of Parenteral and Enteral Nutrition. 27 (6): 419–22. doi:10.1177/0148607103027006419. PMID 14621123.
- ↑ Jung YP, Earnest CP, Koozehchian M, Galvan E, Dalton R, Walker D, et al. (January 2017). "Effects of acute ingestion of a pre-workout dietary supplement with and without p-synephrine on resting energy expenditure, cognitive function and exercise performance". Journal of the International Society of Sports Nutrition. 14: 3. doi:10.1186/s12970-016-0159-2. PMC 5234109. PMID 28096758.
- ↑ Fischer F, Ristow M (May 2020). "Endogenous metabolites promote stress resistance through induction of mitohormesis". EMBO Reports. 21 (5): e50340. doi:10.15252/embr.202050340. PMC 7202198. PMID 32329201.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.