 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1-Chloropiperidine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H10ClN | |
| Molar mass | 119.59 g·mol−1 |
| Appearance | Colorless liquid |
| Boiling point | 50–60 °C (122–140 °F; 323–333 K) 40-50 mm Hg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
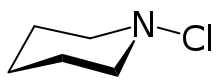
N-Chloropiperidine is the organic compound with the formula C5H10NCl. A colorless liquid, it is a rare example of an organic chloramine, i.e. a compound with an N-Cl bond. It is prepared by treatment of piperidine with calcium hypochlorite. Typical of chloramines, the compound is so reactive that it is generated and used in situ rather than being isolated. The compound undergoes dehydrohalogenation to afford the cyclic imine.[1]
References
- ↑ Claxton, George P.; Allen, Lloyd; Grisar, J. Martin (1977). "2,3,4,5-Tetrahydropyridine Trimer". Organic Syntheses. 56: 118. doi:10.15227/orgsyn.056.0118.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.