 | |
| Names | |
|---|---|
| Preferred IUPAC name
(Phenylimino)-λ4-sulfanone[1] | |
| Other names
N-thionylaniline, phenyliminooxosulfurane, N-sulfinylbenzenamine | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.013.058 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
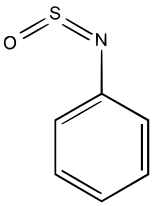
| C6H5NSO | |
| Molar mass | 139.18 |
| Appearance | yellowish oil |
| Density | 1.236 g/mL |
| Boiling point | 88–95 °C (17–20 mm.) |
| Hazards | |
| GHS labelling: | |
  | |
| Danger | |
| H315, H319, H334, H335 | |
| P261, P264, P271, P280, P285, P302+P352, P304+P340, P304+P341, P305+P351+P338, P312, P321, P332+P313, P337+P313, P342+P311, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
N-Sulfinylaniline is the organosulfur compound with the formula C6H5NSO. It is a straw-colored liquid. N-Sulfinylaniline is an example of a sulfinylamine. It is a dienophile[2] and a ligand in organometallic chemistry.[3]
Synthesis and structure
It is prepared by treating aniline with thionyl chloride:[4]
- 3 PhNH2 + SOCl2 → PhNSO + 2 [PhNH3]Cl
X-ray crystallographic analysis confirms that N-sulfinylaniline is structurally related to sulfur dioxide as well as sulfur diimide. The C–S=N=O dihedral angle is –1.60°,[5]
References
- ↑ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 1032. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ↑ Kresze, G.; Wucherpfennig, W. (1967). "New Methods of Preparative Organic Chemistry V: Organic Syntheses with Imides of Sulfur Dioxide". Angewandte Chemie International Edition in English. 6 (2): 149–167. doi:10.1002/anie.196701491. PMID 4962859.
- ↑ Hill, A. F., "Organotransition metallic chemistry of sulfur dioxide analogs", Adv. Organomet. Chem. 1994, 36, 159-227
- ↑ Rajagopalan, S.; Advani, B. G.; Talaty, C. N. (1969). "Diphenylcarbodiimide". Organic Syntheses. 49: 70. doi:10.15227/orgsyn.049.0070..
- ↑ Romano, R.M; Della Védova, C.O; Boese, R. (1999). "A Solid State Study of the Configuration and Conformation of O=S=N–R (R=C6H5 and C6H3(CH3–CH2)2-2,6)". Journal of Molecular Structure. 475 (1): 1–4. Bibcode:1999JMoSt.475....1R. doi:10.1016/S0022-2860(98)00439-6.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.