 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| I3Np | |
| Molar mass | 618 g·mol−1 |
| Appearance | brown solid[1] |
| Density | 6.82 g·cm−3[2] |
| Melting point | 767 °C[1] |
| Structure | |
| orthorhombic | |
| Ccmm (No. 63) | |
| Related compounds | |
Other anions |
neptunium(III) fluoride neptunium(III) chloride neptunium(III) bromide |
Other cations |
uranium(III) iodide plutonium(III) iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Neptunium(III) iodide is the iodide of neptunium with the chemical formula NpI3.
Preparation
Neptunium(III) iodide can be produced by the reaction of neptunium dioxide and aluminium iodide:[3]
- 6 NpO2 + 8 AlI3 → 6 NpI3 + 4 Al2O3 + 3 I2
Properties
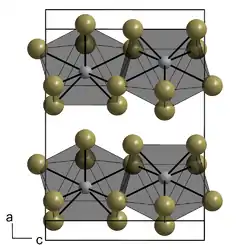
Neptunium(III) iodide is a hygroscopic brown solid with a melting point of 767 °C. It is orthorhombic (plutonium(III) bromide structure), space group Ccmm (No. 63), lattice parameters a = 430pm, b = 1403pm and c = 995pm.[4]
References
- 1 2 Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, p. 1969, ISBN 0-12-352651-5
- ↑ Gmelins Handbuch der anorganischen Chemie, System Nr. 71, Transurane, Teil C, S. 154–155.
- ↑ Georg Brauer (Hrsg.), unter Mitarbeit von Marianne Baudler u. a.: Handbuch der Präparativen Anorganischen Chemie. 3., umgearbeitete Auflage. Band II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3, S. 1269.
- ↑ C. Keller: Die Chemie des Neptuniums. In: Fortschr. chem. Forsch., 1969/70, 13/1, S. 69.
External reading
- Yoshida, Zenko; Johnson, Stephen G.; Kimura, Takaumi; Krsul, John R. (2006), Morss, Lester R.; Edelstein, Norman M.; Fuger, Jean (eds.), "Neptunium", The Chemistry of the Actinide and Transactinide Elements, Dordrecht: Springer Netherlands, pp. 699–812, doi:10.1007/1-4020-3598-5_6, ISBN 978-1-4020-3555-5, retrieved 2023-09-15
- Keller, C. (1969), "Die Chemie des Neptuniums", Anorganische Chemie, Fortschritte der Chemischen Forschung (in German), Berlin/Heidelberg: Springer-Verlag, vol. 13/1, pp. 1–124, doi:10.1007/bfb0051170, ISBN 978-3-540-04488-8, retrieved 2023-09-15
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.