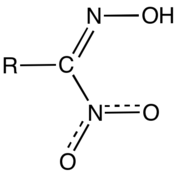
Nitrolic acids are organic compounds with the functional group RC(NO2)=NOH. They are prepared by the reaction of nitroalkanes with base and nitrite sources:[1]
- RCH2NO2 + HNO2 → RC(NO2)=NOH + H2O
The conversion was first demonstrated by Victor Meyer using nitroethane.[2] The reaction proceeds via the intermediacy of the nitronate anion.
Occurrence
Most nitrolic acids are laboratory curiosities. One exception is the compound HO2C(CH2)4C(NO2)=NOH, which is produced by the oxidation of cyclohexanone with nitric acid.[3] This species decomposes to adipic acid and nitrous oxide:
- HO2C(CH2)4C(NO2)=NOH → HO2C(CH2)4CO2H + N2O
This conversion is thought to be the largest anthropogenic route to N2O, which, on a molecule-to-molecule basis, has 298 times the atmospheric heat-trapping ability of carbon dioxide.[4] Adipic acid is a precursor to many nylon polymers. In the end, nitrous oxide is produced in about one to one mole ratio to the adipic acid.[5]
References
- ↑ Matt, C.; Wagner, A.; Mioskowski, C., "Novel Transformation of Primary Nitroalkanes and Primary Alkyl Bromides to the Corresponding Carboxylic Acids", The Journal of Organic Chemistry 1997, 62, 234-235. doi:10.1021/jo962110n
- ↑ Meyer, V.; Locher, J. "Untersuchungen über die Constitution der Nitrolsäuren (Researches on the constitution of the nitrolic acids)" Deut. Chem. Ges. Ber., 1874, volume 7, pp. 670-5. doi:10.1002/cber.187400701211
- ↑ Musser, M. T. (2005). "Adipic Acid". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_269. ISBN 3527306730.
- ↑ "Overview of Greenhouse Gases – Nitrous Oxide" (PDF). US EPA. Page 164 (document header listing). Retrieved 19 March 2014.
- ↑ Parmon, V. N.; Panov, G. I.; Uriarte, A.; Noskov, A. S. (2005). "Nitrous oxide in oxidation chemistry and catalysis application and production". Catalysis Today. Elsevier. 100 (2005): 115–131. doi:10.1016/j.cattod.2004.12.012.