 | |
| Names | |
|---|---|
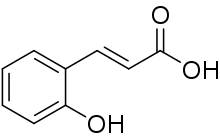
| Preferred IUPAC name
(2E)-3-(2-Hydroxyphenyl)prop-2-enoic acid | |
| Other names
ortho-Coumaric acid 2-Hydroxycinnamic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.009.444 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H8O3 | |
| Molar mass | 164.16 g/mol |
| Hazards | |
| GHS labelling:[1] | |
  | |
| Danger | |
| H301, H315, H319, H335 | |
| P261, P264, P270, P271, P280, P301+P310, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
o-Coumaric acid is a hydroxycinnamic acid, an organic compound that is a hydroxy derivative of cinnamic acid. There are three isomers of coumaric acids — o-coumaric acid, m-coumaric acid, and p-coumaric acid — that differ by the position of the hydroxy substitution of the phenyl group.
Natural occurrence
o-Coumaric acid can be found in vinegar.
2-Coumarate reductase is an enzyme that produces 2-coumarate from 3-(2-hydroxyphenyl)propanoate and NAD+. This enzyme participates in phenylalanine metabolism.[2]
References
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.