 General chemical structure of octabromodiphenyl ethers, where m + n = 8 | |
| Names | |
|---|---|
| Other names
Octabromobiphenyl ether; OctaBDE, Octa-BDE; Octabromodiphenyl oxide; OBDPO | |
| Identifiers | |
| ChemSpider |
|
| ECHA InfoCard | 100.046.428 |
| EC Number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| Properties | |
| C12H2Br8O | |
| Molar mass | 801.379 g·mol−1 |
| Appearance | White solid[1] |
| Density | 2.9 g/cm3[1] |
| Melting point | Depends on product composition[1] |
| Boiling point | Decomposes[1] |
| < 0.1 mg/L[1] | |
| Hazards | |
| GHS labelling: | |
 | |
| Danger | |
| H360Df | |
| Related compounds | |
Related polybrominated diphenyl ethers |
Decabromodiphenyl ether, Pentabromodiphenyl ether |
Related compounds |
diphenyl ether |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Octabromodiphenyl ether (octaBDE, octa-BDE, OBDE, octa, octabromodiphenyl oxide, OBDPO) is a brominated flame retardant which belongs to the group of polybrominated diphenyl ethers (PBDEs).
Composition, uses, and production
Commercial octaBDE (also known as "Octabrom") is a technical mixture of different PBDE congeners having an average of 7.2 to 7.7 bromine atoms per molecule of diphenyl ether.[2] The predominant congeners in commercial octaBDE are those of heptabromodiphenyl ether and octaBDE.[2][3] The term octaBDE alone refers to isomers of octabromodiphenyl ether (PBDE congener numbers 194–205).[4]
| Structure | Congener | Name | Fraction |
|---|---|---|---|
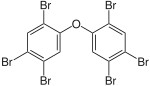 |
BDE-153 | 2,2′,4,4′,5,5′-hexa- bromodiphenyl ether |
0.15–8.7% |
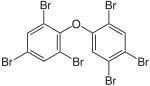 |
BDE-154 | 2,2′,4,4′,5,6′-hexa- bromodiphenyl ether |
0.04–1.1% |
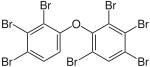 |
BDE-171 | 2,2′,3,3′,4,4′,6-hepta- bromodiphenyl ether |
0.17–1.8% |
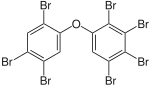 |
BDE-180 | 2,2′,3,4,4′,5,5′-hepta- bromodiphenyl ether |
n.d.–1.7% |
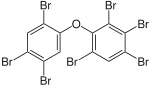 |
BDE-183 | 2,2′,3,4,4′,5′,6-hepta- bromodiphenyl ether |
13–42% |
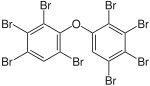 |
BDE-196 | 2,2′,3,3′,4,4′,5,6′-octa- bromodiphenyl ether |
3.1–10.5% |
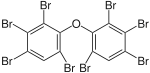 |
BDE-197 | 2,2′,3,3′,4,4′,6,6′-octa- bromodiphenyl ether |
11–22% |
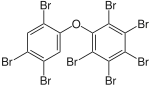 |
BDE-203 | 2,2′,3,4,4′,5,5′,6-octa- bromodiphenyl ether |
4.4–8.1% |
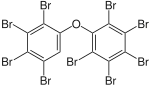 |
BDE-206 | 2,2′,3,3′,4,4′,5,5′,6-nona- bromodiphenyl ether |
1.4–7.7% |
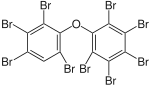 |
BDE-207 | 2,2′,3,3′,4,4′,5,6,6′-nona- bromodiphenyl ether |
11–12% |
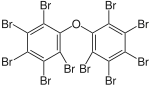 |
BDE-209 | Deca- bromodiphenyl ether |
1.3–50% |
Only congeners with more than 1% listed.
OctaBDE is used in conjunction with antimony trioxide as a flame retardant in the housings of electrical and electronic equipment, mainly in the plastic acrylonitrile butadiene styrene, but also in high impact polystyrene, polybutylene terephthalate and polyamides.[6] Typically 12–15% of the weight of the final product will consist of octaBDE.[6]
The annual demand worldwide was estimated as 3,790 tonnes in 2001, of which Asia accounted for 1,500 tonnes, the Americas 1,500 tonnes, and Europe 610 tonnes.[7] The United Nations Environment Programme reports "Since 2004, it [octaBDE] is no longer produced in the EU, USA and the Pacific Rim and there is no information that indicates it is being produced in developing countries."[3]
Environmental chemistry
OctaBDE is released by different processes into the environment, such as emissions from the manufacture of octaBDE-containing products and from the products themselves.[3] Elevated concentrations can be found in air, water, soil, food, sediment, sludge, and dust.[3][8][9]
In the environment, "photolysis, anaerobic degradation and metabolism in biota" can cause debromination of octaBDE, which produces PBDEs with fewer bromine atoms "which may have higher toxicity and bioaccumulation potential."[3]
Exposures and health effects
OctaBDE may enter the body by ingestion or inhalation.[4] It is "stored mainly in body fat" and may stay in the body for years.[4] In an investigation carried out by the WWF, "the brominated flame retardant chemical (PBDE 153), which is a component of the penta- and octa- brominated diphenyl ether flame retardant products" was found in all blood samples of 14 ministers of health and environment of 13 European Union countries.[10]
The chemical has no proven health effects in humans; however, based on animal experiments, octaBDE may have effects on "the liver, thyroid, and neurobehavioral development."[4]
Governmental actions
The European Union has carried out a comprehensive risk assessment under the Existing Substances Regulation 793/93/EEC.[6] As a consequence, the EU has banned the use of octaBDE since 2004.[11]
In the United States, as of 2005, "no new manufacture or import of" pentaBDE and octaBDE "can occur... without first being subject to EPA [i.e., United States Environmental Protection Agency ] evaluation."[12] As of mid-2007, a total of eleven states in the U.S. had banned octaBDE.[13]
In May 2009, commercial octaBDE was added to the Stockholm Convention as it meets the criteria for the so-called persistent organic pollutants of persistence, bioaccumulation and toxicity.
Alternatives
Alternatives to octaBDE include tetrabromobisphenol A, 1,2-bis (pentabromophenoxy) ethane, 1,2-bis(tribromophenoxy)ethane, triphenyl phosphate, resorcinol bis(diphenylphosphate), and brominated polystyrene; however, for each of these "the existing data on toxicological and ecotoxicological effects are fewer than for octabromodiphenyl ether."[14]
References
- 1 2 3 4 5 Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- 1 2 United States Patent and Trademark Office. Melting point enhancement of partially brominated diphenyl oxide mixtures. US Patent 5,000,879 issued on March 19, 1991.
- 1 2 3 4 5 Ad hoc working group on C-Octabromodiphenyl ether under the Persistent Organic Pollutants Review Committee of the Stockholm Convention. Draft risk profile: commercial octabromodiphenyl ether. United Nations Environment Programme, August 2007.
- 1 2 3 4 Agency for Toxic Substances and Disease Registry. Toxicological Profile for Polybrominated Biphenyls and Polybrominated Diphenyl Ethers (PBBs and PBDEs). Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service, September 2004.
- ↑ M. J. La Guardia, R. C. Hale, E. Harvey: Detailed Polybrominated Diphenyl Ether (PBDE) Congener Composition of the Widely Used Penta-, Octa-, and Deca-PBDE Technical Flame-retardant Mixtures, Environ. Sci. Technol., 2006, 40, 6247–6254, doi:10.1021/es060630m.
- 1 2 3 European Union risk assessment report. Diphenyl ether, octabromo derivative. Luxembourg: Office for Official Publications of the European Communities, 2003. Publication EUR 20403 EN.
- ↑ Bromine Science and Environmental Forum. Major Brominated Flame Retardants Volume Estimates: Total Market Demand By Region in 2001. Archived 2006-11-30 at the Wayback Machine 21 January 2003.
- ↑ Hale RC, La Guardia MJ, Harvey E, Gaylor MO, Mainor TM (2006): Brominated flame retardant concentrations and trends in abiotic media. Chemosphere. 64(2):181-6. doi:10.1016/j.chemosphere.2005.12.006 PMID 16434082
- ↑ Stapleton, Heather M., et al. Polybrominated Diphenyl Ethers in House Dust and Clothes Dryer Lint. Environmental Science & Technology 39(4), 925-931, 2005.
- ↑ WWF Detox Campaign. Bad Blood? A Survey of Chemicals in the Blood of European Ministers. October 2004.
- ↑ Directive 2003/11/Ec of the European Parliament and of the Council of 6 February 2003 amending for the 24th time Council Directive 76/769/EEC relating to restrictions on the marketing and use of certain dangerous substances and preparations (pentabromodiphenyl ether, octabromodiphenyl ether). Official Journal of the European Union 15.2.2003.
- ↑ U.S. Environmental Protection Agency. Polybrominated diphenylethers (PBDEs). "Last updated on Thursday, August 2nd, 2007." Accessed 2007-10-26.
- ↑ Maine Joins Washington, Bans PBDEs. Archived 2007-08-02 at the Wayback Machine Washington, DC: National Caucus of Environmental Legislators, June 18, 2007.
- ↑ Risk & Policy Analysts Limited. Octabromodiphenyl Ether. Risk Reduction Strategy and Analysis of Advantages and Drawbacks. Final Report. Prepared for Department for Environment, Food and Rural Affairs, United Kingdom, June 2002.