 | |
 | |
| Names | |
|---|---|
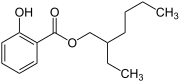
| IUPAC name
2-ethylhexyl 2-hydroxybenzoate | |
| Other names
octisalate; 2-ethylhexyl salicylate; ethyl hexyl salicylate; 2-ethylhexyl ester salicylic acid; salicylic acid, 2-ethylhexyl ester; benzoic acid, 2-hydroxy-, 2-ethylhexyl ester; 2-ethylhexyl ester benzoic acid, 2-hydroxy-; 2-hydroxy- 2-ethylhexyl ester benzoic acid; | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.877 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H22O3 | |
| Molar mass | 250.33 g/mol |
| Density | 1.014 g/cm3 |
| Melting point | < 25 °C (77 °F; 298 K) |
| Boiling point | 189 °C (372 °F; 462 K) |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
2-Ethylhexyl salicylate, or octyl salicylate, is an organic compound used as an ingredient in sunscreens and cosmetics to absorb UVB (ultraviolet) rays from the sun.[1] It is an ester formed by the condensation of salicylic acid with 2-ethylhexanol. It is a colorless oily liquid with a slight floral odor.
The salicylate portion of the molecule absorbs ultraviolet light, protecting skin from the harmful effects of exposure to sunlight. The ethylhexanol portion is a fatty alcohol, adding emollient and oil-like (water resistant) properties.
Safety
Octisalate and all other salicylates have a good safety profile.[2] It is often used to improve affinity and reduce photodegradation of other sunscreen ingredients (such as oxybenzone and avobenzone), and <1% of the applied dose of Octisalate penetrates through the skin. Although Octisalate is a weaker UVB absorber it has superior stability than some other sunscreen active ingredients[3] and does not produce reactive oxygen species when exposed to sunlight. However, it does have some minor sensitization potential; with some individuals experiencing minimal to mild skin irritation.[4]
Notes
- ↑ Skin cancer foundation: Understanding UVA and UVB
- ↑ Rai, Reena; Srinivas, CR (2007). "Photoprotection". Indian Journal of Dermatology, Venereology and Leprology. 73 (2): 73–79. doi:10.4103/0378-6323.31889. PMID 17456910.
- ↑ Ma, Haohua; Wang, Jianqiang; Zhang, Wenpei; Guo, Cheng (2021). "Synthesis of phenylalanine and leucine dipeptide functionalized silica-based nanoporous material as a safe UV filter for sunscreen". Journal of Sol-Gel Science and Technology. 97 (2): 466–478. doi:10.1007/s10971-020-05417-6. S2CID 221937086.
- ↑ Yap, Francis HX; Chua, Hock C.; Tait, Clare P. (10 March 2017). "Active sunscreen ingredients in Australia". Australasian Journal of Dermatology. 58 (4): e160–e170. doi:10.1111/ajd.12597. PMID 28295176. S2CID 32838625.
References
- "The Skin Cancer Foundation's Guide to Sunscreens". Skin Cancer Foundation. 2011. Archived from the original on 23 November 2011. Retrieved 15 November 2011.
