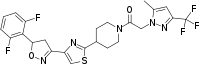
 | |
| Names | |
|---|---|
| Preferred IUPAC name
12,16-Difluoro-75-methyl-73-(trifluoromethyl)-24,25-dihydro-4(4,1)-piperidina-2(5,3)-[1,2]oxazola-3(4,2)-[1,3]thiazola-7(1)-pyrazola-1(1)-benzenaheptaphan-5-one | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.227.885 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C24H22F5N5O2S | |
| Molar mass | 539.53 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Oxathiapiprolin (trade names Orondis,[1] Zorvec, and Segovis) is a fungicide. In the United States, the Environmental Protection Agency has approved it for use against several fungal diseases including downy mildew and various Phytophthora species[1] including late blight on crops including vegetables, ornamentals, and turf.[2]
Its mechanism of action involves binding to the oxysterol-binding protein in Oomycetes.[3][4]
References
- 1 2 "Orondis - Fungicide Product & Label Information". Syngenta US. Retrieved 2021-03-24.
- ↑ "Oxathiapiprolin" (PDF). New Active Ingredient Review. Minnesota Department of Agriculture. October 2015. Archived from the original (PDF) on 2017-11-07. Retrieved 2017-11-03.
- ↑ Cohen, Yigal (2015). "The Novel Oomycide Oxathiapiprolin Inhibits All Stages in the Asexual Life Cycle of Pseudoperonospora cubensis - Causal Agent of Cucurbit Downy Mildew". PLOS ONE. 10 (10): e0140015. Bibcode:2015PLoSO..1040015C. doi:10.1371/journal.pone.0140015. PMC 4599937. PMID 26452052.
- ↑ Pasteris, Robert J; Hanagan, Mary Ann; Bisaha, John J; Finkelstein, Bruce L; Hoffman, Lisa E; Gregory, Vann; Shepherd, Christopher P; Andreassi, John L; Sweigard, James A; Klyashchitsky, Boris A; Henry, Yewande T; Berger, Richard A (2015). "The Discovery of Oxathiapiprolin: A New, Highly-Active Oomycete Fungicide with a Novel Site of Action". Discovery and Synthesis of Crop Protection Products. ACS Symposium Series. Vol. 1204. p. 149. doi:10.1021/bk-2015-1204.ch011. ISBN 978-0-8412-3102-3.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.