| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,3-Oxazole[1] | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 103851 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.474 | ||
| EC Number |
| ||
| 485850 | |||
| MeSH | D010080 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C3H3NO | |||
| Molar mass | 69.06 g/mol | ||
| Density | 1.050 g/cm3 | ||
| Boiling point | 69.5 °C (157.1 °F; 342.6 K) | ||
| Acidity (pKa) | 0.8 (of conjugate acid)[2] | ||
| Hazards | |||
| GHS labelling:[3] | |||
 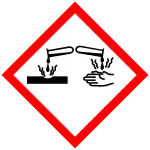 | |||
| Danger | |||
| H225, H318 | |||
| P210, P233, P240, P241, P242, P243, P264+P265, P280, P303+P361+P353, P305+P354+P338, P317, P370+P378, P403+P235, P501 | |||
| Supplementary data page | |||
| Oxazole (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
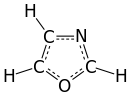
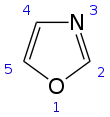
Oxazole is the parent compound for a vast class of heterocyclic aromatic organic compounds. These are azoles with an oxygen and a nitrogen separated by one carbon.[4] Oxazoles are aromatic compounds but less so than the thiazoles. Oxazole is a weak base; its conjugate acid has a pKa of 0.8, compared to 7 for imidazole.
Preparation
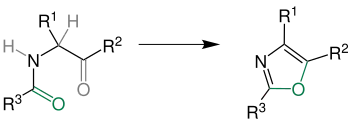
The classic synthetic route the Robinson–Gabriel synthesis by dehydration of 2-acylaminoketones:
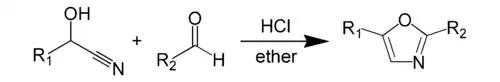
The Fischer oxazole synthesis from cyanohydrins and aldehydes is also widely used:
Other methods are known including the reaction of α-haloketones and formamide and the Van Leusen reaction with aldehydes and TosMIC.
Biosynthesis
In biomolecules, oxazoles result from the cyclization and oxidation of serine or threonine nonribosomal peptides:[5]
 Where X = H, CH
Where X = H, CH
3 for serine and threonine respectively, B = base.
(1) Enzymatic cyclization. (2) Elimination. (3) [O] = enzymatic oxidation.
Oxazoles are not as abundant in biomolecules as the related thiazoles with oxygen replaced by a sulfur atom.
Reactions
With a pKa of 0.8 for the conjugate acid (oxazolium salts), oxazoles are far less basic than imidazoles (pKa = 7). Deprotonation of oxazoles occurs at C2. Formylation with dimethylformamide gives 2-formyloxazole. The lithio compound exists in equilibrium with the ring-opened enolate-isonitrile, which can be trapped by silylation.[4]
Electrophilic aromatic substitution takes place at C5, but requiring electron donating groups.
Nucleophilic aromatic substitution takes place with leaving groups at C2.
Diels–Alder reactions involving oxazole (as dienes) and electrophilic alkenes has been well developed as a route to pyridines. In this way, alkoxy-substituted oxazoles serve a precursors to the pyridoxyl system, as found in vitamin B6. The initial cycloaddition affords a bicyclic intermediate, with an acid-sensitive oxo bridgehead.

In the Cornforth rearrangement of 4-acyloxazoles is a thermal rearrangement reaction with the organic acyl residue and the C5 substituent changing positions.
- Various oxidation reactions. One study[7] reports on the oxidation of 4,5-diphenyloxazole with 3 equivalents of CAN to the corresponding imide and benzoic acid:
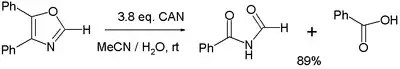
- In the balanced half-reaction three equivalents of water are consumed for each equivalent of oxazoline, generating 4 protons and 4 electrons (the latter derived from CeIV).
See also
- Isoxazole, an analog with the nitrogen atom in position 2.
- Thiazole, an analog with the oxygen replaced by a sulfur.
- Benzoxazole, where the oxazole is fused to a benzene ring.
- Oxazoline, which has one double bond reduced.
- Oxazolidine, which has both double bonds reduced.
- Oxazolone, an analog with a carbonyl group
Additional reading
- Fully Automated Continuous Flow Synthesis of 4,5-Disubstituted Oxazoles Marcus Baumann, Ian R. Baxendale, Steven V. Ley, Christoper D. Smith, and Geoffrey K. Tranmer Org. Lett.; 2006; 8(23) pp 5231 - 5234. doi:10.1021/ol061975c
References
- ↑ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 140. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ↑ Zoltewicz, J. A. & Deady, L. W. Quaternization of heteroaromatic compounds. Quantitative aspects. Adv. Heterocycl. Chem. 22, 71-121 (1978).
- ↑ "Oxazole". pubchem.ncbi.nlm.nih.gov.
- 1 2 T. L. Gilchrist (1997). Heterocyclic Chemistry (3 ed.). Longman. ISBN 0-582-01421-2.
- ↑ Roy, Ranabir Sinha; Gehring, Amy M.; Milne, Jill C.; Belshaw, Peter J.; Walsh, Christopher T.; Roy, Ranabir Sinha; Gehring, Amy M.; Milne, Jill C.; Belshaw, Peter J.; Walsh, Christopher T. (1999). "Thiazole and Oxazole Peptides: Biosynthesis and Molecular Machinery". Natural Product Reports. 16 (2): 249–263. doi:10.1039/A806930A. PMID 10331285.
- ↑ Gérard Moine; Hans-Peter Hohmann; Roland Kurth; Joachim Paust; Wolfgang Hähnlein; Horst Pauling; Bernd–Jürgen Weimann; Bruno Kaesler (2011). "Vitamins, 6. B Vitamins". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.o27_o09. ISBN 978-3-527-30673-2.
- ↑ "Ceric Ammonium Nitrate Promoted Oxidation of Oxazoles", David A. Evans, Pavel Nagorny, and Risheng Xu. Org. Lett.; 2006; 8(24) pp 5669 - 5671; (Letter) doi:10.1021/ol0624530