Phosphoenolpyruvate carboxykinase 2, mitochondrial (PCK2, PEPCK-M), is an isozyme of phosphoenolpyruvate carboxykinase (PCK, PEPCK) that in humans is encoded by the PCK2 gene on chromosome 14. This gene encodes a mitochondrial enzyme that catalyzes the conversion of oxaloacetate (OAA) to phosphoenolpyruvate (PEP) in the presence of guanosine triphosphate (GTP). A cytosolic form of this protein is encoded by a different gene and is the key enzyme of gluconeogenesis in the liver. Alternatively spliced transcript variants have been described.[provided by RefSeq, Apr 2014][5]
Structure
The PCK2 gene encodes the mitochondrial form of PCK and shares a 68% homology in DNA sequence with PCK1 and 70% homology in amino acid sequence with its encoded cytosolic form, PCK1.[6][7] Moreover, PCK2 shares structural homology with PCK1, indicating that the genes originated from a common ancestor gene.[6] Nonetheless, though both genes possess ten exons and nine introns, the sizes of their introns may differ by ~2 kb, with the largest intron in PCK2 spanning 2.5 kb. Altogether, the total length of the PCK2 gene spans ~10 kb. Another difference is the presence of Alu sequences in its introns that are absent in PCK1.[6] PCK2 also contains an 18-residue mitochondrial targeting sequence at its N-terminal.[7] Potential regulatory elements, including five GC boxes and three CCAAT boxes, lie 1819 bp upstream of the transcription start site.[8] In addition, the proximal promoter region of PCK2 contains two putative ATF/CRE sequences which bind ATF4.[9]
Function
As a PCK, PCK2 catalyzes the GTP-driven conversion of OAA to PEP as a rate-limiting step in gluconeogenesis. This conversion step serves as a bridge between glycolytic and TCA cycle intermediates in the mitochondria.[6][9] In pancreatic β-cells, PCK2 regulates glucose-stimulated insulin secretion by recycling GTP generated from the succinyl-CoA synthase reaction. This drives the TCA cycle, converting PEP to pyruvate to acetyl-CoA for the citrate synthase reaction.[9] Since nearly all of the glycolytic reactions upstream of PEP and downstream of glucose-6-phosphate (G6P) are reversible, PCK2-mediated synthesis of PEP could fuel multiple biosynthetic processes, such as serine synthesis, glycerol synthesis, and nucleotide synthesis.[10] Notably, PCK2 preferentially converts OAA derived from lactate and, thus, can promote biosynthesis even under low-glucose conditions.[6][9][10] As a result, PCK2 activity contributes to cell growth and survival during stress.[9]
While PCK1 is mainly expressed in the liver and kidney, PCK2 is ubiquitously expressed in various cell types, including leukocytes and neurons, as well as in non-gluconeogenic tissues, including pancreas, brain, heart. Moreover, while PCK1 expression is regulated by hormones or nutrients involved in gluconeogenesis, PCK2 is constitutively expressed. These differences indicate that PCK2 may also perform non-gluconeogenic functions.[6][9]
Clinical Significance
PCK2 is associated with several cancers, including lung cancer, and promotes tumorigenesis through its gluconeogenic function.[9][10] In low-glucose settings, stress to the endoplasmic reticulum upregulates ATF4, which then upregulates PCK2.[9] As PCK2 allows cells to utilize alternative cataplerotic pathways to convert TCA cycle intermediates to glycolytic intermediates, PCK2 activity can enhance the survival tumor cells facing reduced glucose levels.[9][10]
Due to the gluconeogenic function of PCK2, PCK2 deficiency is expected to disrupt glucose homeostasis and result in hypoglycemia. However, though two cases have been documented, a subsequent study suggested that PCK2 deficiency may not have been the primary cause.[7][11]
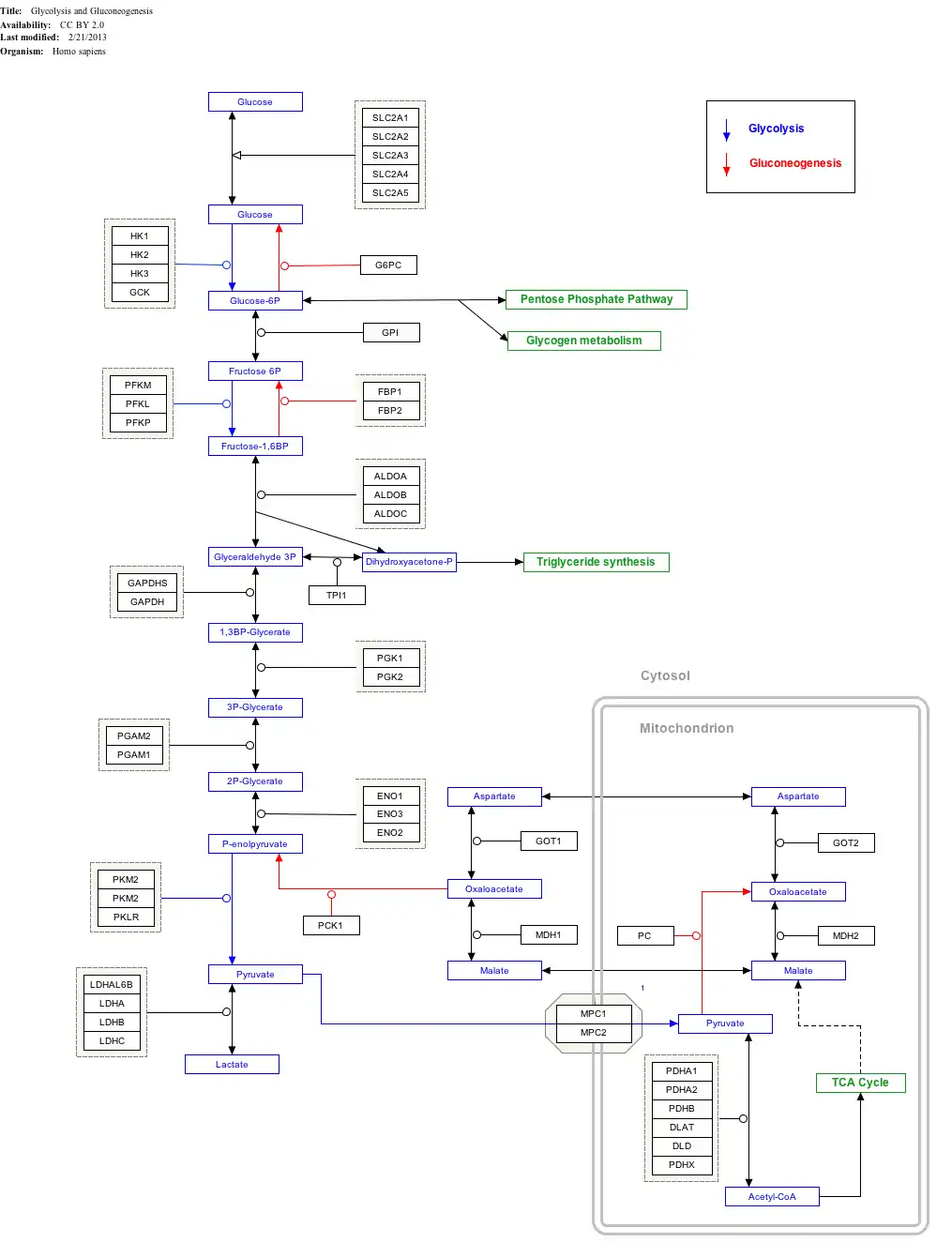
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles.[§ 1]
- ↑ The interactive pathway map can be edited at WikiPathways: "GlycolysisGluconeogenesis_WP534".
See also
References
- 1 2 3 ENSG00000285241 GRCh38: Ensembl release 89: ENSG00000100889, ENSG00000285241 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000040618 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "PCK2 phosphoenolpyruvate carboxykinase 2 (mitochondrial)". NCBI Entrez Gene database.
- 1 2 3 4 5 6 Modaressi S, Brechtel K, Christ B, Jungermann K (July 1998). "Human mitochondrial phosphoenolpyruvate carboxykinase 2 gene. Structure, chromosomal localization and tissue-specific expression". The Biochemical Journal. 333 ( Pt 2) (2): 359–66. doi:10.1042/bj3330359. PMC 1219593. PMID 9657976.
- 1 2 3 Modaressi S, Christ B, Bratke J, Zahn S, Heise T, Jungermann K (May 1996). "Molecular cloning, sequencing and expression of the cDNA of the mitochondrial form of phosphoenolpyruvate carboxykinase from human liver". The Biochemical Journal. 315 ( Pt 3) (3): 807–14. doi:10.1042/bj3150807. PMC 1217278. PMID 8645161.
- ↑ Suzuki M, Yamasaki T, Shinohata R, Hata M, Nakajima H, Kono N (September 2004). "Cloning and reporter analysis of human mitochondrial phosphoenolpyruvate carboxykinase gene promoter". Gene. 338 (2): 157–62. doi:10.1016/j.gene.2004.06.005. PMID 15315819.
- 1 2 3 4 5 6 7 8 9 Méndez-Lucas A, Hyroššová P, Novellasdemunt L, Viñals F, Perales JC (August 2014). "Mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) is a pro-survival, endoplasmic reticulum (ER) stress response gene involved in tumor cell adaptation to nutrient availability". The Journal of Biological Chemistry. 289 (32): 22090–102. doi:10.1074/jbc.M114.566927. PMC 4139223. PMID 24973213.
- 1 2 3 4 Leithner K, Hrzenjak A, Trötzmüller M, Moustafa T, Köfeler HC, Wohlkoenig C, Stacher E, Lindenmann J, Harris AL, Olschewski A, Olschewski H (February 2015). "PCK2 activation mediates an adaptive response to glucose depletion in lung cancer". Oncogene. 34 (8): 1044–50. doi:10.1038/onc.2014.47. PMID 24632615.
- ↑ Samuel VT, Beddow SA, Iwasaki T, Zhang XM, Chu X, Still CD, Gerhard GS, Shulman GI (July 2009). "Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with Type 2 Diabetes". Proceedings of the National Academy of Sciences of the United States of America. 106 (29): 12121–6. doi:10.1073/pnas.0812547106. PMC 2707270. PMID 19587243.