 | |
| Names | |
|---|---|
| IUPAC name
5-Hydroxy-4′,6-dimethoxy-7-[α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranosyloxy]flavone | |
| Systematic IUPAC name
(42S,43R,44S,45S,46R,72R,73R,74R,75R,76S)-25,43,44,45,73,74,75-Heptahydroxy-14,26-dimethoxy-76-methyl-24H-3,6-dioxa-2(2,7)-[1]benzopyrana-4(2,6),7(2)-bis(oxana)-1(1)-benzenaheptaphan-24H-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C29H34O15 | |
| Molar mass | 622.57 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Pectolinarin is a Cirsium isolate with anti-inflammatory activity and similar in chemical structure to linarin.[1]
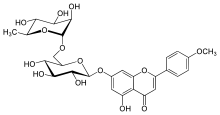
Chemical structure of linarin
External links
- ↑ Lim, H; Son, KH; Chang, HW; Bae, K; Kang, SS; Kim, HP (2008). "Anti-inflammatory activity of pectolinarigenin and pectolinarin isolated from Cirsium chanroenicum". Biological & Pharmaceutical Bulletin. 31 (11): 2063–7. doi:10.1248/bpb.31.2063. PMID 18981574.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.