 | |
 | |
| Names | |
|---|---|
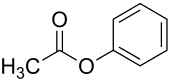
| Preferred IUPAC name
Phenyl acetate | |
| Systematic IUPAC name
Phenyl ethanoate | |
| Other names
Phenol acetate (Acetyloxy)benzene Acetoxybenzene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.004.160 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H8O2 | |
| Molar mass | 136.150 g·mol−1 |
| Density | 1.075 g/mL[1] |
| Melting point | −30 °C (−22 °F; 243 K) |
| Boiling point | 195–196 °C (383–385 °F; 468–469 K)[1] |
| -82.04·10−6 cm3/mol | |
| Hazards | |
| Flash point | 76 °C (169 °F; 349 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Phenyl acetate is the ester of phenol and acetic acid. It can be produced by reacting phenol with acetic anhydride or acetyl chloride.
Phenyl acetate can be separated into phenol and an acetate salt, via saponification: heating the phenyl acetate with a strong base, such as sodium hydroxide, will produce phenol and an acetate salt (sodium acetate, if sodium hydroxide were used).
References
- 1 2 3 Phenyl acetate, Alfa Aesar
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.