 | |
| Names | |
|---|---|
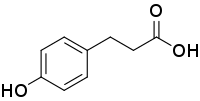
| Preferred IUPAC name
3-(4-Hydroxyphenyl)propanoic acid | |
| Other names
Desaminotyrosine Hydro-p-coumaric acid Phloretate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.007.211 |
| EC Number |
|
| KEGG | |
| MeSH | C008869 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H10O3 | |
| Molar mass | 166.176 g·mol−1 |
| Melting point | 129 °C (264 °F; 402 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Phloretic acid is an organic compound with the formula HOC6H4(CH2)2CO2H. It is a white solid. The compound contains both phenol and carboxylic acid functional groups. It is sometimes called Desaminotyrosine (DAT) because it is identical to the common alpha amino acid tyrosine except for the absence of the amino functional group on the alpha carbon.
Production and occurrence
Phloretic acid is produced by reduction of the unsaturated side chain of p-coumaric acid. Together with phloroglucinol, it is produced by the action of the enzyme phloretin hydrolase on phloretin.
It is found in olives.[1] It is found in the rumen of sheep fed with dried grass.[2] It is also an urinary metabolite of tyrosine in rats.[3]
Polyesters have been prepared from phloretic acid.[4]
It is one of the products of flavonoid metabolism performed by the bacterium Clostridium orbiscindens, a resident of some human guts. [5]
References
- ↑ Owen, R.W; Haubner, R.; Mier, W.; Giacosa, A.; Hull, W.E; Spiegelhalder, B.; Bartsch, H. (2003). "Isolation, structure elucidation and antioxidant potential of the major phenolic and flavonoid compounds in brined olive drupes". Food and Chemical Toxicology. 41 (5): 703–717. doi:10.1016/S0278-6915(03)00011-5. PMID 12659724.
- ↑ Chesson, A; Stewart, CS; Wallace, RJ (1982). "Influence of plant phenolic acids on growth and cellulolytic activity of rumen bacteria". Applied and Environmental Microbiology. 44 (3): 597–603. PMC 242064. PMID 16346090.
- ↑ Booth AN, Masri MS, Robbins DJ, Emerson OH, Jones FT, Deeds F (1960). "Urinary phenolic acid metabolities of tyrosine". Journal of Biological Chemistry. 235 (9): 2649–2652.
- ↑ Reina, Antonio; Gerken, Andreas; Zemann, Uwe; Kricheldorf, Hans R. (1999). "New polymer syntheses, 101. Liquid-crystalline hyperbranched and potentially biodegradable polyesters based on phloretic acid and gallic acid". Macromolecular Chemistry and Physics. 200 (7): 1784–1791. doi:10.1002/(SICI)1521-3935(19990701)200:7<1784::AID-MACP1784>3.0.CO;2-B.
- ↑ Schoefer, Lilian; Mohan, Ruchika; Schwiertz, Andreas; Braune, Annett; Blaut, Michael (2003). "Anaerobic Degradation of Flavonoids by Clostridium orbiscindens". Applied and Environmental Microbiology. 69 (10): 5849–5854. doi:10.1128/AEM.69.10.5849-5854.2003. PMC 201214. PMID 14532034.
External links
 Media related to Phloretic acid at Wikimedia Commons
Media related to Phloretic acid at Wikimedia Commons