 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Phthalazine[2] | |
| Other names
Benzo-orthodiazine 2,3-Benzodiazine Benzo[d]pyridazine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.005.422 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H6N2 | |
| Molar mass | 130.150 g·mol−1 |
| Appearance | Pale yellow needles |
| Melting point | 90 to 91 °C (194 to 196 °F; 363 to 364 K) |
| Boiling point | 315 to 317 °C (599 to 603 °F; 588 to 590 K) (decomposition) |
| Miscible | |
| Acidity (pKa) | 3.39[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
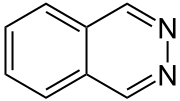
Phthalazine, also called benzo-orthodiazine or benzopyridazine, is a heterocyclic organic compound with the molecular formula C8H6N2. It is isomeric with other naphthyridines including quinoxaline, cinnoline and quinazoline.
Synthesis
Phthalazine can be obtained by the condensation of w-tetrabromorthoxylene with hydrazine, or by the reduction of chlorphthalazine with phosphorus and hydroiodic acid.[4]
Properties
It possesses basic properties and forms addition products with alkyl iodides.[4]
Reactions
Upon oxidation with alkaline potassium permanganate it yields pyridazine dicarboxylic acid. Zinc and hydrochloric acid decompose it with formation of orthoxylylene diamine. The keto-hydro derivative phthalazone (C8H6ON2), is obtained by condensing hydrazine with orthophthalaldehydoacid. On treatment with phosphorus oxychloride, it yields a chlorphthalazine, which with zinc and hydrochloric acid gives isoindole (C8H7N), and with tin and hydrochloric acid, phthalimidine (C8H7ON), the second nitrogen atom being eliminated as ammonia.[4]
References
- ↑ Merck Index, 11th Edition, 7344.
- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 212. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ Brown, H.C., et al., in Baude, E.A. and Nachod, F.C., Determination of Organic Structures by Physical Methods, Academic Press, New York, 1955.
- 1 2 3 One or more of the preceding sentences incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). "Phthalazines". Encyclopædia Britannica. Vol. 21 (11th ed.). Cambridge University Press. p. 545.