 | |
 | |
| Names | |
|---|---|
| IUPAC name
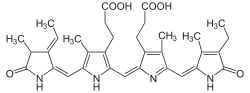
3-[(2Z,5E)-2-[[3-(2-carboxyethyl)-5-[(Z)-[(3R,4R)-3-ethyl-4-methyl-5-oxopyrrolidin-2-ylidene]methyl]-4-methyl-1H-pyrrol-2-yl]methylidene]-5-[(4-ethyl-3-methyl-5-oxopyrrol-2-yl)methylidene]-4-methylpyrrol-3-yl]propanoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| 4285356 | |
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C33H38N4O6 | |
| Molar mass | 586.69 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |

Phycocyanobilin is a blue phycobilin, i.e., a tetrapyrrole chromophore found in cyanobacteria and in the chloroplasts of red algae, glaucophytes, and some cryptomonads. Phycocyanobilin is present only in the phycobiliproteins allophycocyanin and phycocyanin, of which it is the terminal acceptor of energy. It is covalently linked to these phycobiliproteins by a thioether bond.
Phycocyanobilin (PCB), has the ability to bind to human serum albumin (HSA), protein found mainly in the blood of humans. This PCB-HCA complex benefits the structure of HSA, increasing the thermal stability of HSA, as well as increasing its ability to prevent against proteolytic activity of other proteins.[1]
Biosynthetic Pathway
The biosynthetic pathway of phycocyanobilin begins with 5-Aminolevulinic acid (5-ALA).[2] Two molecules of 5-ALA undergo a condensation reaction catalyzed by Porphobilinogen (PBG) Synthase to yield a molecule of Porphobilinogen (PBG) (not shown).[3] Four molecules of PBG are polymerized into a linear tetrapyrrole by Porphobilinogen deaminase. This reaction releases four ammonia molecules in the process. Completion of the tetrapyrrole is performed by Uroporphyrinogen III synthase which results in the macrocyclic Uroporphyrinogen III. Uroporphyrinogen III is then converted to a Heme by a Uroporphyrinogen III decarboxylase. The heme molecule is converted to Biliverdin IX α. Biliverdin is then finally reduced to Phycocyanobilin (PCB) by the Phycocyanin Ferredoxin Oxidoreductase PcyA. Literature circa 1989 includes phytochromobilin as an intermediate in this final conversion.[2]
References
- ↑ Radibratovic M, Minic S, Stanic-Vucinic D, Nikolic M, Milcic M, Cirkovic Velickovic T (2016-12-13). "Stabilization of Human Serum Albumin by the Binding of Phycocyanobilin, a Bioactive Chromophore of Blue-Green Alga Spirulina: Molecular Dynamics and Experimental Study". PLOS ONE. 11 (12): e0167973. Bibcode:2016PLoSO..1167973R. doi:10.1371/journal.pone.0167973. PMC 5154526. PMID 27959940.
- 1 2 Brown, Stanley B.; Houghton, Jennifer D.; Vernon, David I. (1990-04-01). "New trends in photobiology biosynthesis of phycobilins. Formation of the chromophore of phytochrome, phycocyanin and phycoerythrin". Journal of Photochemistry and Photobiology B: Biology. 5 (1): 3–23. doi:10.1016/1011-1344(90)85002-E. ISSN 1011-1344. PMID 2111391.
- ↑ Watanabe, Fumio; Yabuta, Yukinori; Bito, Tomohiro (2014-01-01), Atta-ur-Rahman (ed.), Chapter 11 - Tetrapyrrole Compounds of Cyanobacteria, Studies in Natural Products Chemistry, vol. 42, Elsevier, pp. 341–351, doi:10.1016/b978-0-444-63281-4.00011-2, ISBN 9780444632814, retrieved 2023-06-08
Further reading
- Cole WJ, Chapman DJ, Siegelman HW (1967). "Structure of phycocyanobilin". Journal of the American Chemical Society. 89 (14): 3643–3645. doi:10.1021/ja00990a055. ISSN 0002-7863.