 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,2-Dimethylpropanenitrile[1] | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| 1361449 | |
| ChemSpider | |
| ECHA InfoCard | 100.010.122 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 3273 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H9N | |
| Molar mass | 83.134 g·mol−1 |
| Appearance | Colourless liquid |
| Density | 752 mg mL−1 |
| Melting point | 15 °C (59 °F; 288 K) |
| Boiling point | 106 °C (223 °F; 379 K) |
Refractive index (nD) |
1.3774 |
| Thermochemistry | |
Heat capacity (C) |
179.37 J K−1 mol−1 |
Std molar entropy (S⦵298) |
232.00 J K−1 mol−1 |
Std enthalpy of formation (ΔfH⦵298) |
−39.9 kJ mol−1 |
Std enthalpy of combustion (ΔcH⦵298) |
−3.2146–−3.2132 MJ mol−1 |
| Hazards | |
| GHS labelling: | |
  | |
| Danger | |
| H225, H301, H311, H331 | |
| P210, P261, P280, P301+P310, P311 | |
| Flash point | 4 °C (39 °F; 277 K) |
| Related compounds | |
Related alkanenitriles |
|
Related compounds |
DBNPA |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
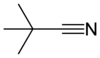
Pivalonitrile is a nitrile with the semi-structural formula (CH3)3CCN, abbreviated t-BuCN. This aliphatic organic compound is a clear, colourless liquid that is used as a solvent and as a labile ligand in coordination chemistry. Pivalonitrile is isomeric with tert-butyl isocyanide but the two compounds do not exist in chemical equilibrium, unlike its silicon analog trimethylsilyl cyanide. [3]
References
- ↑ "Pivalonitrile - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification. Retrieved 8 June 2012.
- ↑ "Pivalonitrile | C5H9N | ChemSpider". www.chemspider.com. Retrieved 19 August 2022.
- ↑ Booth, M. R.; Frankiss, S. G. (1968). "Trimethylsilyl isocyanide". Chem. Commun. (21): 1347–1348. doi:10.1039/C19680001347.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.