 | |
| Identifiers | |
|---|---|
| |
3D model (JSmol) |
|
| |
| |
| Properties | |
| KTcO4 | |
| Molar mass | 201.1 |
| Appearance | colourless solid[1] |
| Melting point | 540 °C |
| Boiling point | around 1000 °C |
| 2.13g (20 °C) | |
| Related compounds | |
Other anions |
potassium permanganate potassium perrhenate |
Other cations |
sodium pertechnetate ammonium pertechnetate silver pertechnetate |
Related compounds |
technetium(VII) oxide pertechnetic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Potassium pertechnetate is a chemical compound of technetium and potassium, with the chemical formula of KTcO4.
Preparation
Potassium pertechnetate can be obtained by the neutralisation of potassium hydroxide and pertechnetic acid:[2]
- KOH + HTcO4 → KTcO4 + H2O
Properties
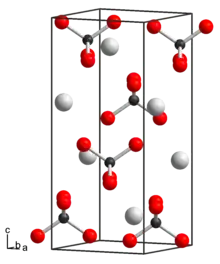
The distance of the Tc–O bond in potassium pertechnetate is 173.9 pm and the angle of the O–Tc–O bond is 108.05° and 110.19°. The distance between the potassium and the oxygen is 289.36 pm and 286 pm.[2] Potassium pertechnetate crystallizes in the tetragonal crystal system with space group I41/a (space group no. 88). The lattice parameters are a = 563.0 pm and c = 1286.7 pm.[3]
Use
Potassium pertechnetate is used to prepare other radiopharmaceuticals.[2]
References
- ↑ 《无机化学丛书》. 第九卷 锰分族 铁系 铂系. 谢高阳 等主编. 科学出版社. 3.13.3 含氧酸及其盐类. P116
- 1 2 3 Weaver, Jamie; Soderquist, Chuck Z.; Washton, Nancy M.; Lipton, Andrew S.; Gassman, Paul L.; Lukens, Wayne W.; Kruger, Albert A.; Wall, Nathalie A.; McCloy, John S. (2017-03-06). "Chemical Trends in Solid Alkali Pertechnetates". Inorganic Chemistry. 56 (5): 2533–2544. doi:10.1021/acs.inorgchem.6b02694. ISSN 0020-1669. PMID 28221786.
- ↑ Klaus Schwochau (Nov 2008), Technetium: Chemistry and Radiopharmaceutical Applications, John Wiley & Sons, p. 129, ISBN 978-3527613373
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.