 | |
| Names | |
|---|---|
| IUPAC name
1-Deoxy-1-(3,3,4,5-tetramethyl-9,11-dioxo-2,3,8,9,10,11-hexahydro-1H,7H-quinolino[1,8-fg]pteridin-7-yl)-D-ribitol 5-(dihydrogen phosphate) | |
| Systematic IUPAC name
(2R,3S,4S)-2,3,4-Trihydroxy-5-(3,3,4,5-tetramethyl-9,11-dioxo-2,3,8,9,10,11-hexahydro-1H,7H-quinolino[1,8-fg]pteridin-7-yl)pentyl dihydrogen phosphate | |
| Other names
prFMN; Prenylated FMNH2 | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| |
| |
| Properties | |
| C22H31N4O9P | |
| Molar mass | 526.483 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Prenylated flavin mononucleotide (prFMN) is a cofactor biosynthesized by the flavin prenyltransferase UbiX and used by UbiD enzymes for reversible decarboxylation reactions. Hence, prFMN is pivotal for catalysis in the ubiquitous microbial UbiD/X system.[1]
prFMN is flavin prenylated at the N5 and C6 positions resulting in the formation of a fourth non-aromatic ring.[2]
prFMN was discovered in 2015 at the University of Manchester by David Leys' group.[2][3]
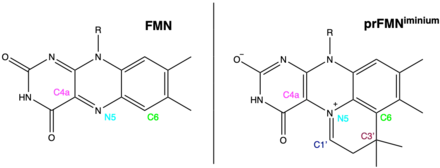 Structure of FMN and prFMNiminium. The leftmost structure shows FMN (Flavin mononucleotide), the rightmost structure shows prFMNiminium (prenylated flavin mononucleotide), Note the presence of the quaternary carbon (C3’) in prFMNiminium indicative of cofactor prenylation.
Structure of FMN and prFMNiminium. The leftmost structure shows FMN (Flavin mononucleotide), the rightmost structure shows prFMNiminium (prenylated flavin mononucleotide), Note the presence of the quaternary carbon (C3’) in prFMNiminium indicative of cofactor prenylation.
Two studies in 2015 characterized UbiX as a flavin prenyltransferase, supplying prFMN to UbiD/Fdc1 which utilises the cofactor to catalyse a reversible decarboxylation reaction.[2][3] Ferulic acid decarboxylase (Fdc1) from A. niger co-expressed in E.coli with UbiX from E.coli (AnFdc1UbiX) once purified had clear spectral differences to singly expressed AnFdc1, and was capable of in vitro decarboxylation of a range of aromatic carboxylic acids. The atomic resolution of the crystal structure of AnFdc1UbiX, allowed elucidation of the structure of the modified FMN cofactor classified as prFMN. The crystal structure revealed an isopentenyl-adduct to the N5-C6 of FMN, with the modifications branched nature and the position of the covalent linkages with flavin suggesting prenylation.
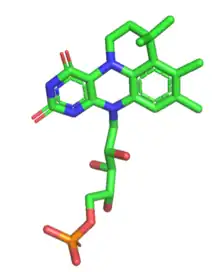 prFMNiminium in the active site of AnFDC1. PDB file: 4ZA4.
prFMNiminium in the active site of AnFDC1. PDB file: 4ZA4.
PrFMNOx
UbiD activation by UbiX/prFMN was found to be dependent on oxygen suggesting that the reduced prFMN product of UbiX is oxidised to the catalytically relevant form. Several variations of the oxidised prFMN (prFMNox) cofactor were observed: prFMNiminium, hydroxylated prFMNiminium and prFMNketimine. Determination of the prFMN isomer that was catalytically relevant involved incubation of AnFdc1UbiX with phenylpyruvate (of which a small proportion is α-hydroxycinnamic acid which closely resembles cinnamic acid - a model substrate). Incubation with phenylpyruvate lead to an altered UV-Vis spectrum and reversible enzyme inhibition. The crystal structure of AnFdc1UbiX with phenylpyruvate revealed a bond between C1’ of prFMNiminium and a phenylacetaldehyde adduct – a species that can be formed by decarboxylation of α-hydroxycinnamic acid and tautomerization of the α-hydroxystyrene prFMNiminium adduct.
This observation confirmed that it is the prFMNiminium that is the catalytically relevant cofactor.
References
- ↑ Leys, David (December 2018). "Flavin metamorphosis: cofactor transformation through prenylation". Current Opinion in Chemical Biology. 47: 117–125. doi:10.1016/j.cbpa.2018.09.024. PMID 30326424.
- 1 2 3 White, Mark D.; Payne, Karl A. P.; Fisher, Karl; Marshall, Stephen A.; Parker, David; Rattray, Nicholas J. W.; Trivedi, Drupad K.; Goodacre, Royston; Rigby, Stephen E. J.; Scrutton, Nigel S.; Hay, Sam; Leys, David (17 June 2015). "UbiX is a flavin prenyltransferase required for bacterial ubiquinone biosynthesis". Nature. 522 (7557): 502–506. Bibcode:2015Natur.522..502W. doi:10.1038/nature14559. PMC 4988493. PMID 26083743.
- 1 2 Payne, Karl A. P.; White, Mark D.; Fisher, Karl; Khara, Basile; Bailey, Samuel S.; Parker, David; Rattray, Nicholas J. W.; Trivedi, Drupad K.; Goodacre, Royston; Beveridge, Rebecca; Barran, Perdita; Rigby, Stephen E. J.; Scrutton, Nigel S.; Hay, Sam; Leys, David (17 June 2015). "New cofactor supports α,β-unsaturated acid decarboxylation via 1,3-dipolar cycloaddition". Nature. 522 (7557): 497–501. Bibcode:2015Natur.522..497P. doi:10.1038/nature14560. PMC 4988494. PMID 26083754.