 | |
 | |
| Names | |
|---|---|
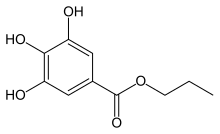
| IUPAC name
Propyl 3,4,5-trihydroxybenzoate | |
| Other names
Gallic acid, propyl ester n-Propyl gallate E310 | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.090 |
| EC Number |
|
| E number | E310 (antioxidants, ...) |
| MeSH | Propyl+Gallate |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H12O5 | |
| Molar mass | 212.20 g/mol |
| Appearance | White crystalline powder |
| Melting point | 150 °C (302 °F; 423 K) |
| Boiling point | Decomposes |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Propyl gallate, or propyl 3,4,5-trihydroxybenzoate is an ester formed by the condensation of gallic acid and propanol. Since 1948, this antioxidant has been added to foods containing oils and fats to prevent oxidation.[1] As a food additive, it is used under the E number E310.
Description
Propyl gallate is an antioxidant. It protects against oxidation by hydrogen peroxide and oxygen free radicals. It appears as a white to creamy-white crystalline odorless solid.[2][3]
Production
Propyl gallate does not occur naturally, and is prepared either from reactions with gallic acid and 1-propanol, or by enzyme catalysis of tannic acid.[4] Syntheses with gallic acid have been the most prominent methods of production, and include Steglich esterification with N,N'-diisopropylcarbodiimide and 4-dimethylaminopyridine, anhydrous addition of thionyl chloride, and Fischer esterification with various catalysts.[5]
Uses
Propyl gallate is used to protect oils and fats in products from oxidation; it is used in foods, cosmetics, hair products, adhesives, biodiesel, and lubricants.[6] It is often used interchangeably with octyl gallate and dodecyl gallate in these applications.[3]
It is used as a triplet state quencher and an antioxidant in fluorescence microscopy.[7]
Biological effects
A 1993 study in fat rodents found little or no effect on carcinogenesis by propyl gallate.[8]
A 2009 study found that propyl gallate acts as an estrogen antagonist.[9]
References
- ↑ "Final Report on the Amended Safety Assessment of Propyl Gallate". International Journal of Toxicology. 26 (suppl. 3): 89–118. 2007. doi:10.1080/10915810701663176. ISSN 1091-5818. PMID 18080874. S2CID 39562131.
- ↑ Gálico, D. A.; Nova, C. V.; Guerra, R. B.; Bannach, G. (2015-09-01). "Thermal and spectroscopic studies of the antioxidant food additive propyl gallate". Food Chemistry. 182: 89–94. doi:10.1016/j.foodchem.2015.02.129. ISSN 0308-8146.
- 1 2 EFSA Panel on Food additives and Nutrient Sources added to Food (ANS) (2014). "Scientific Opinion on the re‐evaluation of propyl gallate (E 310) as a food additive". EFSA Journal. 12 (4). doi:10.2903/j.efsa.2014.3642.
- ↑ Nie, Guangjun; Liu, Hui; Chen, Zhen; Wang, Peng; Zhao, Genhai; Zheng, Zhiming (2012). "Synthesis of propyl gallate from tannic acid catalyzed by tannase from Aspergillus oryzae: Process optimization of transesterification in anhydrous media". Journal of Molecular Catalysis. 82: 102–108. doi:10.1016/j.molcatb.2012.06.003. ISSN 1381-1177.
- ↑ Nguyen, Van Hai; Le, Minh Ngoc; Nguyen, Hoa Binh; Ha, Kieu Oanh; Pham, Thai Ha Van; Nguyen, Thi Hong; Dao, Nguyet Suong Huyen; Nguyen, Van Giang; Nguyen, Dinh Luyen; Trinh, Nguyen Trieu (2021-04-12). "Propyl Gallate". Molbank. 2021 (2): M1201. doi:10.3390/M1201. ISSN 1422-8599.
- ↑ Hosseinzadeh-Bandbafha, Homa; Kumar, Dipesh; Singh, Bhaskar; Shahbeig, Hossein; Lam, Su Shiung; Aghbashlo, Mortaza; Tabatabaei, Meisam (2022-07-01). "Biodiesel antioxidants and their impact on the behavior of diesel engines: A comprehensive review". Fuel Processing Technology. 232: 107264. doi:10.1016/j.fuproc.2022.107264. ISSN 0378-3820.
- ↑ Jerker Widengren; Andriy Chmyrov; Christian Eggeling; Per-Åke Löfdahl & Claus A. M. Seidel (2007). "Strategies to Improve Photostabilities in Ultrasensitive Fluorescence Spectroscopy". The Journal of Physical Chemistry A. 111 (3): 429–440. Bibcode:2007JPCA..111..429W. doi:10.1021/jp0646325. PMID 17228891.
- ↑ Hirose, Masao, et al. "Modification of carcinogenesis by α-tocopherol, t-butylhydro-quinone, propyl gallate and butylated hydroxytoluene in a rat multi-organ carcinogenesis model." Carcinogenesis 14.11 (1993): 2359-2364.
- ↑ Alessio Amadasi; Andrea Mozzarelli; Clara Meda; Adriana Maggi; Pietro Cozzini (2009). "Identification of Xenoestrogens in Food Additives by an Integrated in Silico and in Vitro Approach". Chem. Res. Toxicol. 22 (1): 52–63. doi:10.1021/tx800048m. PMC 2758355. PMID 19063592.