| |||
| Names | |||
|---|---|---|---|
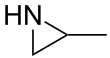
| IUPAC name
2-Methylaziridine | |||
| Other names
1,2-Propylenimine | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.000.799 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1921 (inhibited) | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C3H7N | |||
| Molar mass | 57.096 g·mol−1 | ||
| Appearance | Colorless, oily liquid[1] | ||
| Odor | ammonia-like[1] | ||
| Density | 0.9 g/mL[2] | ||
| Melting point | −63 °C (−81 °F; 210 K)[2] | ||
| Boiling point | 67 °C (153 °F; 340 K)[2] | ||
| Miscible[2] | |||
| Vapor pressure | 112 mmHg (20°C)[1] | ||
| Hazards | |||
| GHS labelling: | |||
     | |||
| Danger | |||
| H225, H300, H310, H318, H330, H350, H411 | |||
| P201, P202, P210, P233, P240, P241, P242, P243, P260, P262, P264, P270, P271, P273, P280, P281, P284, P301+P310, P302+P350, P303+P361+P353, P304+P340, P305+P351+P338, P308+P313, P310, P320, P321, P322, P330, P361, P363, P370+P378, P391, P403+P233, P403+P235, P405, P501 | |||
| Flash point | −4 °C (25 °F; 269 K)[2] | ||
| Lethal dose or concentration (LD, LC): | |||
LCLo (lowest published) |
500 ppm (rat, 4 hr)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 2 ppm (5 mg/m3) [skin][1] | ||
REL (Recommended) |
Ca TWA 2 ppm (5 mg/m3) [skin][1] | ||
IDLH (Immediate danger) |
Ca [100 ppm][1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Propyleneimine (or propylene imine) is the organic compound with the formula CH3CH(NH)CH2. It is a secondary amine and the smallest chiral aziridine (ring containing C2N). It is a flammable colorless liquid. Its derivatives, copolymers and oligomers, are of commercial interest.[4]
Uses
This chemical is used in the paper, textile, rubber and pharmaceutical industries. Propyleneimine is also used in making paint.
The top global producers of this specialty chemical include DuPont, Mitsubishi Chemical Holdings Corporation, Sigma-Aldrich, Dixie Chemical Company, J and K Scientific, Apollo Scientific, Mitsui Chemicals.[5]
The compound is also of interest for the synthesis of dendrimers, a process that exploits the tendency of aziridines to undergo ring-opening reactions.[6] [2]
Health Effects
NIOSH considers propyleneimine a potential occupational carcinogen.
The Dixie Chemical Company facility in the Bayport Industrial District, Pasadena, Texas, has been noted for releasing toxic air pollution.[7]
References
- 1 2 3 4 5 6 NIOSH Pocket Guide to Chemical Hazards. "#0537". National Institute for Occupational Safety and Health (NIOSH).
- 1 2 3 4 5 6 Propyleneimine International Chemical Safety Card at actrav.itcilo.org
- ↑ "Propylene imine". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ↑ Steuerle, Ulrich; Feuerhake, Robert (2006). "Aziridines". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_239.pub2. ISBN 978-3527306732.
- ↑ Global QYResearch. "Propyleneimine Market Analysis, Size, Share, Trends, Growth and Forecasts Report 2019-2026". MarketWatch, Inc. Retrieved 14 March 2020.
- ↑ Malik, N.; Wiwattanapatapee, R.; Klopsch, R.; Lorenz, K.; Frey, H.; Weener, J. W.; Meijer, E. W.; Paulus, W.; Duncan, R. (2000). "Dendrimers: Relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labeled polyamidoamine dendrimers in vivo". Journal of Controlled Release. 65 (1–2): 133–148. doi:10.1016/S0168-3659(99)00246-1. PMID 10699277.
- ↑ "Breath to the People: Sacred Air and Toxic Pollution" (PDF). United Church of Christ. p. 12, 21. Retrieved 14 March 2020.