 | |
| Names | |
|---|---|
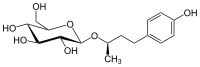
| IUPAC name
(2R)-4-(4-Hydroxyphenyl)butan-2-yl β-D-glucopyranoside | |
| Systematic IUPAC name
(2R,3S,4S,5R,6R)-2-(Hydroxymethyl)-6-{[(2R)-4-(4-hydroxyphenyl)butan-2-yl]oxy}oxane-3,4,5-triol | |
| Other names
Betuloside (-)-Rhododendrin | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H24O7 | |
| Molar mass | 328.361 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Rhododendrin (betuloside) is an arylbutanoid glycoside and a phenylpropanoid, a type of natural phenol. It can be found in the leaves of Rhododendron aureum[1] or in Cistus salviifolius.[2]
In vitro, it shows analgesic, anti-inflammatory[1] and diuretic[3] properties.
References
- 1 2 Kim, M. H.; Nugroho, A.; Choi, J.; Park, J. H.; Park, H. J. (2011). "Rhododendrin, an analgesic/anti-inflammatory arylbutanoid glycoside, from the leaves of Rhododendron aureum". Archives of Pharmacal Research. 34 (6): 971–978. doi:10.1007/s12272-011-0614-1. PMID 21725818. S2CID 19606824.
- ↑ Danne, A.; Petereit, F.; Nahrstedt, A. (1994). "Flavan-3-ols, prodelphinidins and further polyphenols from Cistus salvifolius". Phytochemistry. 37 (2): 533–538. doi:10.1016/0031-9422(94)85094-1. PMID 7765630.
- ↑ Zhang, B; Li, JB; Zhang, DM; Ding, Y; Du, GH (2007). "Analgesic and anti- inflammatory activities of a fraction rich in gaultherin isolated from Gaultheria yunnanensis (Franch.)". Pharmacognosy Reviews. 30 (10): 465–469. doi:10.4103/0973-7847.91102. PMC 3263046. PMID 22279370.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.