 | |
| Names | |
|---|---|
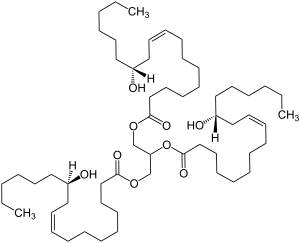
| Systematic IUPAC name
Propane-1,2,3-triyl tris[(9Z,12R)-12-hydroxyoctadec-9-enoate] | |
| Other names
Glycerin triricinoleate; Glycerol triricinoleate; Glyceryl triricinoleate; Ricinoleic acid triglyceride; Ricinoleic triglyceride; Triricinolein | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.018.016 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C57H104O9 | |
| Molar mass | 933.450 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Ricinolein is the chief constituent of castor oil and is the triglyceride of ricinoleic acid.[1] Castor oil, the expressed natural fatty oil of the seeds of Ricinus communis also contains mixtures of the glycerides of isoricinoleic acids and much smaller traces of tristearin and the glyceride of dihydroxysteric acid. Ricinolein is the active principle in the use of castor oil as a purgative and solvent for several medically useful alkaloids.
References
- ↑ Achaya, K. T.; Saletore, S. A. (1952). "Triricinolein and ricinoleic acid from castor oil". Journal of Scientific & Industrial Research. 11B (11): 471–474.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.