| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Hydroxybenzaldehyde[1] | |||
| Other names
Salicylaldehyde Salicylic aldehyde o-Hydroxybenzaldehyde | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 471388 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.783 | ||
| EC Number |
| ||
| 3273 | |||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C7H6O2 | |||
| Molar mass | 122.123 g·mol−1 | ||
| Density | 1.146 g/cm3 | ||
| Melting point | −7 °C (19 °F; 266 K) | ||
| Boiling point | 196 to 197 °C (385 to 387 °F; 469 to 470 K) | ||
| -64.4·10−6 cm3/mol | |||
| Hazards[2] | |||
| GHS labelling: | |||
  | |||
| Warning | |||
| H302, H315, H317, H319, H335, H411 | |||
| P280, P305+P351+P338 | |||
| Safety data sheet (SDS) | [2] | ||
| Related compounds | |||
Related compounds |
Salicylic acid Benzaldehyde Salicylaldoxime | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
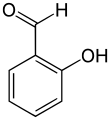
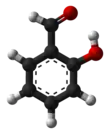
Salicylic aldehyde (2-hydroxybenzaldehyde) is an organic compound with the formula C6H4OH(CHO).[3][4] Along with 3-hydroxybenzaldehyde and 4-hydroxybenzaldehyde, it is one of the three isomers of hydroxybenzaldehyde. This colorless oily liquid has a bitter almond odor at higher concentration. Salicylaldehyde is a precursor to coumarin and a variety of chelating agents.
Production
Salicylaldehyde is produced by condensation of phenol with formaldehyde to give hydroxybenzyl alcohol, which is oxidized to the aldehyde.[4] Salicylaldehydes in general are prepared by ortho-selective formylation reactions from the corresponding phenol, for instance by the Duff reaction, Reimer–Tiemann reaction, or by treatment with paraformaldehyde in the presence of magnesium chloride and a base.[5]
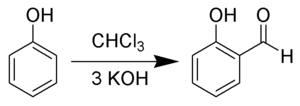
Salicylaldehyde can also be prepared from phenol and chloroform in a Reimer–Tiemann reaction:[6]
Natural occurrences
Salicylaldehyde was identified as a characteristic aroma component of buckwheat.[7]
It is also one of the components of castoreum, the exudate from the castor sacs of the mature North American beaver (Castor canadensis) and the European beaver (Castor fiber), used in perfumery.
Furthermore, salicylaldehyde occurs in the larval defensive secretions of several leaf beetle species that belong the subtribe Chrysomelina.[8] An example for a leaf beetle species that produces salicylaldehyde is the red poplar leaf beetle Chrysomela populi.
Reactions and applications
Salicylaldehyde is mainly used commercially as a precursor to coumarin.[4]

- Oxidation with hydrogen peroxide gives catechol (1,2-dihydroxybenzene) (Dakin reaction).[9]
- Etherification with chloroacetic acid followed by cyclisation gives the heterocycle benzofuran (coumarone).[10] The first step in this reaction to the substituted benzofuran is called the Rap–Stoermer condensation after E. Rap (1895) and R. Stoermer (1900).[11][12]
- Salicylaldehyde is converted to chelating ligands by condensation with amines. With ethylenediamine, it condenses to give the ligand salen. Hydroxylamine gives salicylaldoxime.
- Condensation with diethyl malonate gives 3-carbethoxycoumarin (a derivative of coumarin) by an aldol condensation.[13]
Internal hydrogen bonding
Due to the ortho positioning of the hydroxy- and aldehyde groups, an internal hydrogen bond is formed between the groups. The hydroxy group serves here as the hydrogen bond donor, and the aldehyde as hydrogen bond acceptor. This internal hydrogen is not found in the other hydroxybenzaldehyde isomers. When the aldehyde is reacted with an amine to form an imine, the internal hydrogen bond is even stronger.[14] In addition, tautomerisation further increases the stability of the compound.[15] The internal hydrogen bond also ensures that the aldehyde (or corresponding imine) is held into the same plane, making the whole molecule essentially flat.[16]
References
- ↑ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 652. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- 1 2 Sigma-Aldrich Co., Salicylaldehyde. Retrieved on 2018-05-24.
- ↑ Merck Index, 11th Edition, 8295
- 1 2 3 Maliverney, Christian; Mulhauser, Michel (2000). "Hydroxybenzaldehydes". Kirk-Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.0825041813011209.a01. ISBN 978-0-471-48494-3.
- ↑ Trond Vidar Hansen; Lars Skattebøl (2005). "Ortho-Formylation of Phenols; Preparation of 3-Bromosalicylaldehyde". Organic Syntheses. 82: 64. doi:10.15227/orgsyn.089.0220.
- ↑ Brühne, F.; Wright, E. "Benzaldehyde". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_463.pub2. ISBN 978-3527306732.
{{cite encyclopedia}}: CS1 maint: multiple names: authors list (link) - ↑ Janeš, D.; Kreft, S. (2008). "Salicylaldehyde is a characteristic aroma component of buckwheat groats". Food Chemistry. 109 (2): 293–298. doi:10.1016/j.foodchem.2007.12.032. PMID 26003350.
- ↑ Pauls, G., Becker, T., et al. (2016). Two Defensive Lines in Juvenile Leaf Beetles; Esters of 3-nitropropionic Acid in the Hemolymph and Aposematic Warning. Journal of Chemical Ecology 42 (3) 240-248.
- ↑ Dakin, H. D. (1923). "Catechol" (PDF). Organic Syntheses. 3: 28.; Collective Volume, vol. 1, p. 149
- ↑ Burgstahler, A. W.; Worden, L. R. (1966). "Coumarone". Organic Syntheses. 46: 28. doi:10.15227/orgsyn.046.0028.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Rap, E. (November 1895). "Sull' α-Benzoilcumarone" [On the α-Benzoylcoumaron]. Gazzetta Chimica Italiana. 2 (4): 285–290.
- ↑ Stoermer, R. (1900). "Synthesen und Abbaureactionen in der Cumaronreihe". Liebig's Annalen der Chemie. 312 (3): 237–336. doi:10.1002/jlac.19003120302.
- ↑ Horning, E. C.; Horning, M. G.; Dimmig, D. A. (1948). "3-Carbethoxycoumarin". Organic Syntheses. 28: 24. doi:10.15227/orgsyn.028.0024.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Schoustra, S.K.; Asadi, V.; Zuilhof, H.; Smulders, M.M.J. (2023). "Internal hydrogen bonding of imines to control and enhance the dynamic mechanical properties of covalent adaptable networks". European Polymer Journal. 195: 112209. doi:10.1016/j.eurpolymj.2023.112209.
- ↑ Metzler, C.M.; Cahill, A.; Metzler, D.E. (1980). "Equilibriums and absorption spectra of Schiff bases". J. Am. Chem. Soc. 102 (19): 6075–6082. doi:10.1021/ja00539a017.
- ↑ Kandambeth, S.; Shinde, D.B; Panda, M.K.; Lukose, B.; Heine, T.; Banerjee, R. (2013). "Enhancement of Chemical Stability and Crystallinity in Porphyrin-Containing Covalent Organic Frameworks by Intramolecular Hydrogen Bonds". Angew. Chem. Int. Ed. 52 (49): 13052–13056. doi:10.1002/anie.201306775.