 | |
| Names | |
|---|---|
| IUPAC name
3,4-diaminocyclobut-3-ene-1,2-dione | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H4N2O2 | |
| Molar mass | 112.088 g·mol−1 |
| Appearance | white solid |
| Melting point | 338–340 °C (640–644 °F; 611–613 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Squaramide is the organic compound with the formula O2C4(NH2)2. Not an amide in the usual sense, it is a derivative of squaric acid wherein the two OH groups are replaced by NH2 groups. Squaramides refer to a large class of derivatives wherein some of the H's are replaced by organic substituents. Exploiting their rigid planar structures, these compounds are of interest as hydrogen-bond donors in supramolecular chemistry and organocatalysis.[1] Squaramides exhibit 10-50x greater affinity for halides than do thioureas.[2]
Squaramide is prepared by ammonolysis of diesters of squaric acid:[3][4]
- O2C4(OEt)2 + 2 NH3 → O2C4(NH2)2 + 2 EtOH
N-Substituted squaramides are prepared similarly, using amines in place of ammonia.
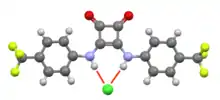
Chloride-squaramide interaction in O2C4(NH(C6H4CF3)2. The characteristic planarity of a squaramide is evident.[2]
References
- ↑ Chauhan, P.; Mahajan, S.; Kaya, U.; Hack, D.; Enders, D. (2015). "Bifunctional Amine-Squaramides: Powerful Hydrogen-Bonding Organocatalysts for Asymmetric Domino/Cascade Reactions". Adv. Synth. Catal. 357 (2–3): 253–281. doi:10.1002/adsc.201401003.
- 1 2 Nathalie Busschaert; Isabelle L. Kirby; Sarah Young; Simon J. Coles; Peter N. Horton; Mark E. Light; Philip A. Gale (2012). "Squaramides as Potent Transmembrane Anion Transporters". Angew. Chem. Int. Ed. 51 (18): 4426–4430. doi:10.1002/anie.201200729. PMID 22461434. S2CID 34164978.
- ↑ Storer, R. Ian (2013). "Squaramide". e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn01531. ISBN 978-0-471-93623-7.
- ↑ Ian Storer, R.; Aciro, Caroline; Jones, Lyn H. (2011). "Squaramides: Physical properties, synthesis and applications". Chemical Society Reviews. 40 (5): 2330–2346. doi:10.1039/c0cs00200c. PMID 21399835.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.