Sukbok Chang | |
|---|---|
장석복 | |
 | |
| Born | August 1, 1962 |
| Alma mater | Korea University, KAIST, Harvard University |
| Awards | Top Scientist and Technologist Award of Korea (2019), Highly Cited Researcher (2015-2019), Korea Science Award (2013) |
| Scientific career | |
| Institutions | Caltech, Ewha Womans University, KAIST, Institute for Basic Science |
| Korean name | |
| Hangul | |
| Hanja | |
| Revised Romanization | Jang Seokbok |
| McCune–Reischauer | Chang Sŏkpok |
Sukbok Chang (장석복; born August 1, 1962) is a South Korean organic chemist. He is a distinguished professor in the Department of Chemistry at Korea Advanced Institute of Science and Technology (KAIST). He is also the director of the Institute for Basic Science (IBS) Center for Catalytic Hydrocarbon Functionalizations (CCHF). He was an associate editor on ACS Catalysis and has served on the editorial advisory boards of The Journal of Organic Chemistry, Journal of the American Chemical Society, and Accounts of Chemical Research.[2] His major research interest is transition metal catalyzed C-H bond functionalization for the carbon-carbon bond and carbon-heteroatom bond formation.
Career
Sukbok Chang received his B.S degree from Korea University in 1985, and M.S degree from KAIST in 1987. Then, he joined Eric N. Jacobsen’s group and received his PhD in 1996 at Harvard University. He subsequently worked with Robert H. Grubbs at Caltech as a postdoctoral fellow from 1996 to 1998. In early 1998, he joined the faculty of Ewha Womans University as an assistant professor, and moved to KAIST as a full professor in 2002. In 2012, he was selected as a director of the Center for Catalytic Hydrocarbon Functionalizations at the Institute for Basic Science, which is the biggest Korean government funded research institute. He also has been working as an associate editor of the journal ACS Catalysis since 2015. In 2023, he was selected to co-run the KAIST Cross Generation Creation Lab, a laboratory designed to continue the know-how of professors about to retire through collaboration with younger professors.[3][4][5]
Major contributions
Chang’s group studies new organic reactions and mechanisms with transition metal catalysis.[6] In particular, his group contributed to the development of “copper catalyzed multicomponent coupling” in the 2000s. Since 2008, his group has focused on C-H functionalization and made a number of contributions.[7]
Copper-catalyzed multicomponent coupling
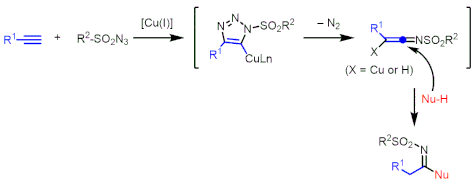
Cu-catalyzed multicomponent coupling is a notable process developed by Chang's group. In 2005, they published a highly efficient and mild catalytic three component coupling between an alkyne, sulfonyl azide, and amine.[8] Unlike click chemistry which generates 1,4-triazoles as products, in this case a Cu(I) catalyst, sulfonyl azide and alkyne generate ketenimine intermediate after releasing N2 gas. This electrophilic ketenimine intermediate reacts with amines and to generate asymmetric imines as products. Chang's group also showed water,[9] alcohols,[10] the C3 position of pyrrole[11] and other nucleophiles can be used in this reaction.
Rhodium, Iridium-catalyzed C-N bond formation

Rhodium or iridium catalyzed C-H amidation and amination are other achievements of his group. In 2012, his group published rhodium catalyzed intermolecular amidation of arenes using sulfonyl azide as a nitrene precursor.[12] This reaction generates N2 as the single byproduct, doesn't need external oxidant, has broad substrate scope and high functional group tolerance. Chang's group advanced their work by using different directing groups,[13] different azides[14] and various substrates.[15] They also published that iridium also works well for C-H amidation/amination.[16][17][18]

In 2016, Chang's group discovered new nitrogen sources.[19] Their new nitrene precursor, 1,4,2-dioxazol-5-one, is more convenient to prepare, store and use compared to azides. Moreover, it has a strong affinity to the rhodium or Iridium metal center, and thus gives excellent amidation efficiency.[20] They later published selective formation of gamma-lactams via C-H amidation[21][22] with this type of nucleophile.
Honors and awards
- 2022: Ho-Am Prize in Science[23]
- 2019: Top Scientist and Technologist Award of Korea, Korean Federation of Science and Technology Societies (ko)[2][24][25]
- 2018: Korea Toray Science Award, Korea Toray Science Foundation
- 2018: JSPS Invitational Fellowship, Japan Society for the Promotion of Science
- 2018: Grand Academic Research Award, KAIST
- 2017: 1st ACS-KCS Excellence Award, American Chemical Society and Korean Chemical Society[26][27]
- 2017: Humboldt Research Award, Alexander von Humboldt Foundation
- 2016: Yoshida Prize, International Organic Chemistry Foundation[28][29]
- 2015-2020: Highly Cited Researcher in chemistry, Clarivate Analytics[30][31]
- 2015: Knowledge Creation Award, Ministry of Science, ICT and Future Planning
- 2014: Member of the Korean Academy of Science and Technology[32]
- 2013: Korea Science Award
- 2013: Kyung-Ahm Prize, Kyung-Ahm Education & Cultural Foundation
- 2010: KCS Academic Award, Korean Chemical Society
- 2008: Star Faculty, Korea Research Foundation
- 2006: One of 50 Representative Research Performances, Korea Science & Engineering Foundation
- 2005: Shim Sang Cheol Award, Korean Chemical Society
- 2003: Thieme Journals Award, Synlett/Synthesis Professorship Award in Asian Area
- 2002: Young Chemist Award, Korean Chemical Society sponsored by WILEY
References
- ↑ "회원: 장석복 (張碩福)". Korean Academy of Science and Technology (in Korean). Retrieved 6 March 2020.
- 1 2 Fields-Hall, Mia (5 November 2019). "Sukbok Chang Wins Top Korean Science Prize". ACS Axial. American Chemical Society. Retrieved 6 March 2020.
- ↑ 노현섭 (11 January 2023). ""학문의 대 잇는다"…KAIST '초세대 협업연구실' 추가 개소". 서울경제 (in Korean). Retrieved 11 January 2023.
- ↑ 여용준 (11 January 2023). "KAIST, 초세대 협업연구실 2곳 추가 개소". 글로벌이코노믹 (in Korean). Retrieved 11 January 2023.
- ↑ 한성형 (11 January 2023). "카이스트 김정호·장석복 교수팀, 학문의 대를 잇는 '초세대 협업연구실' 추가 개소". Korea Lecturer News (in Korean). Retrieved 11 January 2023.
- ↑ "Sukbok Chang's group website".
- ↑ Cho, Seung Hwan; Hwang, Seung Jun; Chang, Sukbok (July 2008). "Palladium-Catalyzed C−H Functionalization of PyridineN-Oxides: Highly Selective Alkenylation and Direct Arylation with Unactivated Arenes". Journal of the American Chemical Society. 130 (29): 9254–9256. doi:10.1021/ja8026295. ISSN 0002-7863. PMID 18582040.
- ↑ Bae, Imhyuck; Han, Hoon; Chang, Sukbok (February 2005). "Highly Efficient One-Pot Synthesis ofN-Sulfonylamidines by Cu-Catalyzed Three-Component Coupling of Sulfonyl Azide, Alkyne, and Amine". Journal of the American Chemical Society. 127 (7): 2038–2039. doi:10.1021/ja0432968. ISSN 0002-7863. PMID 15713069.
- ↑ Cho, Seung Hwan; Yoo, Eun Jeong; Bae, Imhyuck; Chang, Sukbok (November 2005). "Copper-Catalyzed Hydrative Amide Synthesis with Terminal Alkyne, Sulfonyl Azide, and Water". Journal of the American Chemical Society. 127 (46): 16046–16047. doi:10.1021/ja056399e. ISSN 0002-7863. PMID 16287290.
- ↑ Yoo, Eun Jeong; Ahlquist, Mårten; Bae, Imhyuck; Sharpless, K. Barry; Fokin, Valery V.; Chang, Sukbok (July 2008). "Mechanistic Studies on the Cu-Catalyzed Three-Component Reactions of Sulfonyl Azides, 1-Alkynes and Amines, Alcohols, or Water: Dichotomy via a Common Pathway". The Journal of Organic Chemistry. 73 (14): 5520–5528. doi:10.1021/jo800733p. ISSN 0022-3263. PMID 18557650.
- ↑ Cho, Seung Hwan; Chang, Sukbok (2008-03-31). "Room Temperature Copper-Catalyzed 2-Functionalization of Pyrrole Rings by a Three-Component Coupling Reaction". Angewandte Chemie International Edition. 47 (15): 2836–2839. doi:10.1002/anie.200705940. ISSN 1433-7851. PMID 18318034.
- ↑ Kim, Ji Young; Park, Sae Hume; Ryu, Jaeyune; Cho, Seung Hwan; Kim, Seok Hwan; Chang, Sukbok (2012-05-24). "Rhodium-Catalyzed Intermolecular Amidation of Arenes with Sulfonyl Azides via Chelation-Assisted C–H Bond Activation". Journal of the American Chemical Society. 134 (22): 9110–9113. doi:10.1021/ja303527m. ISSN 0002-7863. PMID 22624801.
- ↑ "Graphical Abstract: Angew. Chem. Int. Ed. 39/2012". Angewandte Chemie International Edition. 51 (39): 9709–9721. 2012-09-19. doi:10.1002/anie.201290068. hdl:2027.42/137365. ISSN 1433-7851.
- ↑ Shin, Kwangmin; Baek, Yunjung; Chang, Sukbok (2013-06-20). "Direct CH Amination of Arenes with Alkyl Azides under Rhodium Catalysis". Angewandte Chemie International Edition. 52 (31): 8031–8036. doi:10.1002/anie.201302784. ISSN 1433-7851. PMID 23788328.
- ↑ "Rhodium-Catalyzed Direct Amination of Arene C-H Bonds Using Azides as the Nitrogen Source". Organic Syntheses. 91: 52. 2014. doi:10.15227/orgsyn.091.0052. ISSN 0078-6209.
- ↑ Lee, Donggun; Kim, Youngchan; Chang, Sukbok (2013-10-10). "Iridium-Catalyzed Direct Arene C–H Bond Amidation with Sulfonyl- and Aryl Azides". The Journal of Organic Chemistry. 78 (21): 11102–11109. doi:10.1021/jo4019683. ISSN 0022-3263. PMID 24079849.
- ↑ Kim, Jinwoo; Chang, Sukbok (2014-01-27). "Iridium-Catalyzed Direct CH Amidation with Weakly Coordinating Carbonyl Directing Groups under Mild Conditions". Angewandte Chemie International Edition. 53 (8): 2203–2207. doi:10.1002/anie.201310544. ISSN 1433-7851. PMID 24470125.
- ↑ Kang, Taek; Kim, Youngchan; Lee, Donggun; Wang, Zhen; Chang, Sukbok (2014-03-05). "Iridium-Catalyzed Intermolecular Amidation of sp3 C–H Bonds: Late-Stage Functionalization of an Unactivated Methyl Group". Journal of the American Chemical Society. 136 (11): 4141–4144. doi:10.1021/ja501014b. ISSN 0002-7863. PMID 24580093.
- ↑ Park, Yoonsu; Park, Kyung Tae; Kim, Jeung Gon; Chang, Sukbok (2015-03-30). "Mechanistic Studies on the Rh(III)-Mediated Amido Transfer Process Leading to Robust C–H Amination with a New Type of Amidating Reagent". Journal of the American Chemical Society. 137 (13): 4534–4542. doi:10.1021/jacs.5b01324. ISSN 0002-7863. PMID 25789561.
- ↑ Park, Yoonsu; Heo, Joon; Baik, Mu-Hyun; Chang, Sukbok (2016-10-13). "Why is the Ir(III)-Mediated Amido Transfer Much Faster Than the Rh(III)-Mediated Reaction? A Combined Experimental and Computational Study". Journal of the American Chemical Society. 138 (42): 14020–14029. doi:10.1021/jacs.6b08211. ISSN 0002-7863. PMID 27690406.
- ↑ Hong, Seung Youn; Park, Yoonsu; Hwang, Yeongyu; Kim, Yeong Bum; Baik, Mu-Hyun; Chang, Sukbok (2018-03-01). "Selective formation of γ-lactams via C–H amidation enabled by tailored iridium catalysts". Science. 359 (6379): 1016–1021. Bibcode:2018Sci...359.1016H. doi:10.1126/science.aap7503. ISSN 0036-8075. PMID 29496875.
- ↑ Park, Yoonsu; Chang, Sukbok (2019-02-18). "Asymmetric formation of γ-lactams via C–H amidation enabled by chiral hydrogen-bond-donor catalysts". Nature Catalysis. 2 (3): 219–227. doi:10.1038/s41929-019-0230-x. ISSN 2520-1158. S2CID 104461199.
- ↑ 이인준 (31 May 2022). "32회 삼성호암상…오용근 포스텍 교수 등 6인 수상". Newsis (in Korean). Retrieved 3 June 2022.
- ↑ "2019 Korea Best Scientist and Technologist Award, President of Korea". IBS KAIST Center for Catalytic Hydrocarbon Functionalizations. 4 July 2019. Retrieved 6 March 2020.
- ↑ "'최고과기인상'에 김기남 삼성전자·장석복 KAIST". Naver (in Korean). HelloDD. 2 July 2019. Retrieved 6 March 2020.
- ↑ "Professor Chang, Sukbok received the ACS-KCS Excellence Award for 2017". Department of Chemistry. KAIST. 27 April 2017. Retrieved 6 March 2020.
- ↑ "American and Korean Chemical Societies collaborate to recognize advancement of science". American Chemical Society. 24 April 2017. Retrieved 6 March 2020.
- ↑ "Awards". Department of Chemistry. KAIST. Retrieved 6 March 2020.
- ↑ "장석복 KAIST 화학과 교수". Nobel Science (in Korean). 18 October 2018. Retrieved 6 March 2020.
- ↑ "IBS Places First Among Korean Institutions by Featuring 9 Scientists in List of Highly Cited Researchers". Institute for Basic Science. 4 December 2018. Retrieved 6 March 2020.
- ↑ "Seven IBS Scientists Named World's Most Highly Cited Researchers: Accounting for 13.1% of Korea's scientists on the list". Institute for Basic Science. 20 November 2019. Retrieved 6 March 2020.
- ↑ "한림원 사람들/회원: 회원동정 (장석복)". Korean Academy of Science and Technology (in Korean). 4 July 2019. Retrieved 6 March 2020.