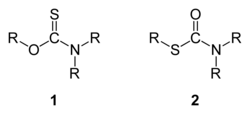
In organic chemistry, thiocarbamates (thiourethanes) are a family of organosulfur compounds. As the prefix thio- suggests, they are sulfur analogues of carbamates. There are two isomeric forms of thiocarbamates: O-thiocarbamates, ROC(=S)NR2 (esters), and S-thiocarbamates, RSC(=O)NR2 (thioesters).
Synthesis
Thiocarbamates can be synthesised by the reaction of water or alcohols upon thiocyanates (Riemschneider thiocarbamate synthesis):[1][2]
- RSCN + H2O → RSC(=O)NH2
- RSCN + R'OH → RSC(=O)NR'H
Similar reactions are seen between alcohols and thiocarbamoyl chlorides such as dimethylthiocarbamoyl chloride; as well as between thiols and cyanates.[2] The herbicide Cycloate is produced in this way:
- C6H11(C2H5)NCOCl + C2H5SH → C6H11(C2H5)NCOSC2H5 + HCl
Other related thiocarbamate herbicides include vernolate (C3H7)2NCOSC3H7 and triallate ((i−C3H7)2NCOSCH2CCl=CCl2.[3]
Salts of thiocarbamate arise by the reaction of amines with carbonyl sulfide:
- 2 R2NH + COS → [R2NH+2][R2N−COS−]
Reactions and occurrence
In the Newman-Kwart rearrangement O-thiocarbamates can isomerise to S-thiocarbamates.[5] This reaction, which generally requires high temperatures, is an important method for the synthesis of thiophenols.

See also
- Dithiocarbamate
- Carbamate
- Tolnaftate, a thiocarbamate used as an antifungal agent
References
- ↑ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, p. 1269, ISBN 978-0-471-72091-1
- 1 2 Walter, W.; Bode, K.-D. (April 1967). "Syntheses of Thiocarbamates". Angewandte Chemie International Edition in English. 6 (4): 281–293. doi:10.1002/anie.196702811.
- ↑ Appleby, Arnold P.; Müller, Franz; Carpy, Serge (2001). "Weed Control". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a28_165. ISBN 3-527-30673-0.
- ↑ McMillan M, Spinks EA, Fenwick GR (January 1986). "Preliminary Observations on the Effect of Dietary Brussels Sprouts on Thyroid Function". Hum Toxicol. 5 (1): 15–19. doi:10.1177/096032718600500104. PMID 2419242.
- ↑ Newman, Melvin S.; Hetzel, Frederick W. (1971). "Thiophenols from Phenols: 2-Naphthalenethiol". Org. Synth. 51: 139. doi:10.15227/orgsyn.051.0139.