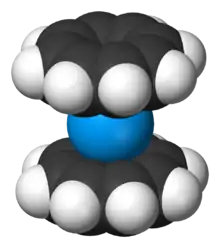
Actinocenes are a family of organoactinide compounds consisting of metallocenes containing elements from the actinide series. They typically have a sandwich structure with two dianionic cyclooctatetraenyl ligands (COT2-, which is C
8H2−
8) bound to an actinide-metal center (An) in the oxidation state IV, resulting in the general formula An(C8H8)2.[1][2]
Characterised actinocenes
| Name | Formula | AnIV centre | First synthesis | Crystal colour | An–COT distance (Å) | Space group |
|---|---|---|---|---|---|---|
| Thorocene | Th(C8H8)2 | Th | 1969 | bright yellow | 2.004 | P21/n |
| Protactinocene | Pa(C8H8)2 | Pa | 1974 | yellowish | – | P21/n |
| Uranocene | U(C8H8)2 | U | 1968 | deep green | 1.926 | P21/n |
| Neptunocene | Np(C8H8)2 | Np | 1970 | yellow-brown | 1.909 | P21/n |
| Plutonocene | Pu(C8H8)2 | Pu | 1970 | dark red | 1.898 | I2/m |
The most studied actinocene is uranocene, U(C8H8)2, which in 1968 was the first member of this family to be synthesised and is still viewed as the archetypal example.[2][3] Other actinocenes that have been synthesised are protactinocene[4] (Pa(C8H8)2), thorocene[5] (Th(C8H8)2), neptunocene[6] (Np(C8H8)2), and plutonocene[7][8] (Pu(C8H8)2). Especially the latter two, neptunocene and plutonocene, have not been extensively studied experimentally since the 1980s because of the radiation hazard they pose.[7][8]
Bonding
The actinide-cyclooctatetraenyl bonding has been of interest for multiple theoretical studies.[8][9] Computational chemistry methods indicate bonding with a large covalent character resulting mainly from the mixing of actinide 6d orbitals with ligand π-orbitals, with a smaller interaction involving the actinide 5f and ligand π-orbitals.[9] The covalent component is characterised by donation of electron density to the actinide.
Analogous sandwiched M(C8H8)2 compounds also exist for lanthanides M = Nd, Tb, and Yb, but therein the bonding is mostly ionic rather than covalent (see lanthanocenes).[3]
See also
References
- ↑ Minasian, Stefan G.; Keith, Jason M. (2014). "New evidence for 5f covalency in actinocenes determined from carbon K-edge XAS and electronic structure theory". Chem. Sci. 5 (1): 351–359. doi:10.1039/C3SC52030G.
- 1 2 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements. Butterworth-Heinemann. pp. 1278–1280. ISBN 978-0-08-037941-8.
- 1 2 Seyferth, D. (2004). "Uranocene. The First Member of a New Class of Organometallic Derivatives of the f Elements". Organometallics. 23 (15): 3562–3583. doi:10.1021/om0400705.
- ↑ Goffart, J.; Fuger, J.; Brown, D.; Duyckaerts, G. (1974-05-01). "On the cyclooctatetraenyl compounds of actinide elements part II. Bis-(cyclooctatetraenyl) protactinium(IV)". Inorganic and Nuclear Chemistry Letters. 10 (5): 413–419. doi:10.1016/0020-1650(74)80119-4. ISSN 0020-1650.
- ↑ Avdeef, Alex; Raymond, Kenneth N.; Hodgson, Keith O.; Zalkin, Allan (1972-05-01). "Two isostructural actinide .pi. complexes. Crystal and molecular structure of bis(cyclooctatetraenyl)uranium(IV), U(C8H8)2, and bis(cyclooctatetraenyl)thorium(IV), Th(C8H8)2". Inorganic Chemistry. 11 (5): 1083–1088. doi:10.1021/ic50111a034. ISSN 0020-1669.
- ↑ De Ridder, D. J. A.; Rebizant, J.; Apostolidis, C.; Kanellakopulos, B.; Dornberger, E. (1996-03-15). "Bis(cyclooctatetraenyl)neptunium(IV)". Acta Crystallographica Section C: Crystal Structure Communications. 52 (3): 597–600. doi:10.1107/S0108270195013047. ISSN 0108-2701.
- 1 2 Windorff, Cory J.; Sperling, Joseph M.; Albrecht-Schönzart, Thomas E.; Bai, Zhuanling; Evans, William J.; Gaiser, Alyssa N.; Gaunt, Andrew J.; Goodwin, Conrad A. P.; Hobart, David E.; Huffman, Zachary K.; Huh, Daniel N. (2020-09-21). "A Single Small-Scale Plutonium Redox Reaction System Yields Three Crystallographically-Characterizable Organoplutonium Complexes". Inorganic Chemistry. 59 (18): 13301–13314. doi:10.1021/acs.inorgchem.0c01671. ISSN 0020-1669. OSTI 1680020. PMID 32910649. S2CID 221623763.
- 1 2 3 Apostolidis, Christos; Walter, Olaf; Vogt, Jochen; Liebing, Phil; Maron, Laurent; Edelmann, Frank T. (2017-04-24). "A Structurally Characterized Organometallic Plutonium(IV) Complex". Angewandte Chemie International Edition. 56 (18): 5066–5070. doi:10.1002/anie.201701858. PMC 5485009. PMID 28371148.
- 1 2 Kerridge, Andrew (2014). "f-Orbital covalency in the actinocenes (An = Th–Cm): multiconfigurational studies and topological analysis" (PDF). RSC Advances. 4 (24): 12078–12086. Bibcode:2014RSCAd...412078K. doi:10.1039/C3RA47088A.