| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
N,N-Diphenylaniline | |||
| Other names
Triphenylamine N,N,N-Triphenylamine N,N-Diphenylbenzeneamine | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.009.123 | ||
| EC Number |
| ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C18H15N | |||
| Molar mass | 245.32 g/mol | ||
| Appearance | Off-white solid | ||
| Density | 0.774 g/cm3 | ||
| Melting point | 127 °C (261 °F; 400 K) | ||
| Boiling point | 347 to 348 °C (657 to 658 °F; 620 to 621 K) | ||
| Almost insoluble | |||
| log P | 5.74 | ||
| Hazards | |||
| GHS labelling: | |||
 | |||
| Warning | |||
| H315, H319 | |||
| P264, P280, P302+P352, P305+P351+P338, P321, P332+P313, P337+P313, P362 | |||
| Flash point | 180 °C (356 °F; 453 K) open cup | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
none[1] | ||
REL (Recommended) |
TWA 5 mg/m3[1] | ||
IDLH (Immediate danger) |
N.D.[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
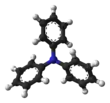
Triphenylamine is an organic compound with formula (C6H5)3N. In contrast to most amines, triphenylamine is nonbasic. At room temperature it appears as a colorless crystalline solid, with monoclinic structure. It is well miscible in diethyl ether and benzene, but it is practically insoluble in water, and partially in ethanol. Its derivatives have useful properties in electrical conductivity and electroluminescence, and they are used in OLEDs as hole-transporters.[2]
Triphenylamine can be prepared by arylation of diphenylamine.[3]
Chemical properties
Triphenylamine has three aromatic groups directly linked to the central nitrogen atom. Each aromatic group acts as an electron attractor, directing the electron cloud of the lone pair of nitrogen towards it. With the delocalization of the nitrogen lone pair,[4] a partial positive charge is conferred to nitrogen, counterbalanced by the partial negative charge localized on the aromatic groups. This arrangement prevents nitrogen protonation, a key mechanism for providing basicity to a solution.
From this characteristic, moreover, it follows that the three N-C bonds all lie on the same plane and that they are located at 120° from each other, which is not the case with aliphatic amines and ammonia, where the orbitals of nitrogen are arranged in a tetrahedron. Due to steric hindrance, the phenyl groups are not on the same plane defined by the three N-C bonds, but are twisted, giving the molecule its characteristic "propeller-like" shape.[5]
See also
References
- 1 2 3 NIOSH Pocket Guide to Chemical Hazards. "#0643". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Wei Shi, Suqin Fan, Fei Huang, Wei Yang, Ransheng Liu and Yong Cao "Synthesis of Novel Triphenylamine-based Conjugated Polyelectrolytes and Their Application to Hole-Transport Layer in Polymeric Light-Emitting Diodes" J. Mater. Chem., 2006, 16, 2387-2394. doi:10.1039/B603704F
- ↑ F. D. Hager "Triphenylamine" Org. Synth. 1928, 8, 116. doi:10.15227/orgsyn.008.0116
- ↑ T. Zhang, I. E. Brumboiu, C. Grazioli, A. Guarnaccio, M. Coreno, M. de Simone, A. Santagata, H. Rensmo, B. Brena, V. Lanzilotto, and C. Puglia "Lone-Pair Delocalization Effects within Electron Donor Molecules: The Case of Triphenylamine and Its Thiophene-Analog" J. Phys. Chem. C 2018, 122, 17706−17717. doi:10.1021/acs.jpcc.8b06475
- ↑ Sobolev, A. N.; Belsky, V. K.; Romm, I. P.; Chernikova, N. Yu.; Guryanova, E. N. (1985). "Structural investigation of the triaryl derivatives of the Group V elements. IX. Structure of triphenylamine, C18H15N". Acta Crystallographica Section C Crystal Structure Communications. 41 (6): 967–971. doi:10.1107/S0108270185006217.