| Triticeae | |
|---|---|
.jpg.webp) | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Angiosperms |
| Clade: | Monocots |
| Clade: | Commelinids |
| Order: | Poales |
| Family: | Poaceae |
| Clade: | BOP clade |
| Subfamily: | Pooideae |
| Supertribe: | Triticodae |
| Tribe: | Triticeae L. |
| Genera | |
|
See text. | |
Triticeae is a botanical tribe within the subfamily Pooideae of grasses that includes genera with many domesticated species. Major crop genera found in this tribe include wheat (see wheat taxonomy), barley, and rye; crops in other genera include some for human consumption, and others used for animal feed or rangeland protection. Among the world's cultivated species, this tribe has some of the most complex genetic histories. An example is bread wheat, which contains the genomes of three species with only one being a wheat Triticum species. Seed storage proteins in the Triticeae are implicated in various food allergies and intolerances.
Genera of Triticeae
Genera recognized in Triticeae according to Robert Soreng et al.:[1]
- Aegilops
- Agropyron
- Amblyopyrum
- Anthosachne
- Australopyrum
- Connorochloa
- Crithopsis
- Dasypyrum
- Douglasdeweya
- Elymus (syn. Campeiostachys, Elytrigia, Hystrix, Roegneria, Sitanion)
- Eremopyrum
- Festucopsis
- Henrardia
- Heteranthelium
- Hordelymus
- Hordeum (syn. Critesion)
- Kengyilia
- Leymus (syn. Aneurolepidium, Eremium, Macrohystrix, Microhystrix)
- Pascopyrum
- Peridictyon
- Psathyrostachys
- Pseudoroegneria
- Secale
- Stenostachys
- Taeniatherum
- Thinopyrum
- Triticum
Cultivated or edible species

Aegilops
- Various species (rarely identifiable to species in archaeological material) occur in pre-agrarian archaeobotanical remains from Near Eastern sites. Their edible grains were doubtless harvested as wild food resources.
- speltoides - ancient food grain, putative source of B genome in bread wheat and G genome in T. timopheevii
- tauschii - Source of D genome in wheat
Amblyopyrum
- muticum - Source of T genome.
Elymus
Various species are cultivated for pastoral purposes or to protect fallow land from opportunistic or invasive species
Hordeum
Many barley cultivars
- vulgare - common barley (6 subspecies, ~100 cultivars)
- bulbosum - edible seeds
- murinum (mouse barley) - cooked as piñole, bread-flour capable, medicinal: diuretic.
Leymus
- arenarius (Lyme grass) - bread-flour capable, possible food additive
- racemosus (Volga Wild Rye) - drought tolerant cereal, used in Russia
- condensatus (Giant Wild Rye) - Edible seeds, harvesting problematic small seeds
- triticoides (Squaw grass) - used in North America, seed hairs must be singed
Secale
Ryes
- cereale (Cereal Rye) - Livestock feed and sour dough bread - 6 subspecies.
- cornutum (spurred rye) - herbal medicine: ergot (ergot of spurred rye) at very low doses; [2] dangerously toxic as food.
- strictum - actively cultivated
- sylvestre - (Tibetan Rye) - actively cultivated in Tibet and China highlands.
- vavilovii (Armenian Wild Rye) - edible seeds, thickener.
Triticum
(Wheat)
- aestivum (bread wheat) - (AABBDD Genome)
- compactum (club wheat)
- macha (hulled)
- spelta (hulled, spelt)
- sphaerococcum (shot wheat)
- monococcum (Einkorn wheat) (A Genome)
- timopheevii (Sanduri wheat)
- turgidum (poulard wheat) (AABB Genome)[3]
- carthlicum (Persian black wheat)
- dicoccoides (wild emmer wheat)
- dicoccum (cultivated emmer wheat) - used to make Farro
- durum (durum wheat)
- paleocolchicum
- polonicum (Polish wheat)
- turanicum
Genetics
| Genera & Species | 1st | 2nd | 3rd | ||
| Triticum boeoticum | AA | ||||
| Triticum monococcum | AMAM | ||||
| Triticum urartu | AUAU | ||||
| Aegilops speltoides var. speltoides | BB | ||||
| Aegilops caudata | CC | ||||
| Aegilops tauschii | DD | ||||
| Lophopyrum elongatum | EE | ||||
| Hordeum vulgare | HH | ||||
| Thinopyrum bessarabicum | JJ | ||||
| Aegilops comosa | MM | ||||
| Aegilops uniaristata | NN | ||||
| Henrardia persica | OO | ||||
| Agropyrum cristatum | PP | ||||
| Secale cereale | RR | ||||
| Aegilops bicornis | SS | ||||
| Amblyopyrum muticum | TT | ||||
| Aegilops umbellulata | UU | ||||
| Dasypyrum | VV | ||||
| Psathyrostachys | NsNs | ||||
| Pseudoroegneria | StSt | ||||
| Triticum zhukovskyi | AA | AMAM | GG | ||
| Triticum turgidum | AA | BB | |||
| Triticum aestivum | AA | BB | DD | ||
| Triticum timopheevii | AA | GG | |||
| Aegilops cylindrica | CC | DD | |||
| Stenostachys sp. | HH | WW | |||
| Elmyus canadensis | HH | StSt | |||
| Elmyus abolinii | YY | StSt | |||
| Thinopyrum Vjd =(V/J/D) | JJ | StSt | VjdVjd | ||
| Leymus tricoides | NsNs | XmXm |
Triticeae and its sister tribe Bromeae (bromes or cheat grasses) when joined form a sister clade with Poeae and Aveneae (Oats). Inter-generic gene flow characterized these taxa from the early stages. For example, Poeae and Aveneae share a mtDNA genetic marker with barley and 10 other members of Triticeae, whereas all 19 genera of Triticeae bear a wheat marker along with Bromeae.[4]
Genera within Triticeae contain diploid, allotetraploid and/or allohexaploid genomes, the capacity to form allopolyploid genomes varies within the tribe. In this tribe, the majority of diploid species tested are closely related to Aegilops, the more distal members (earliest branch points) include Hordeum (Barley), Eremian, Psathyrostachys. The broad distribution of cultivars within the Tribe and the properties of the proteins have implication in the treatment of certain digestive diseases and autoimmune disorders.
Evolution of the tribe
One of the earliest branches in Triticeae, to Pseudoroegeneria, produces the genome StSt and another Hordeum then genome = HH. Allotetraploid combinations of Pseudoroegeneria and Hordeum and are seen in Elmyus (HHStSt),[5] but also shows introgression from Australian and Agropyron wheatgrasses.[6] Elymus contains mostly Pseudoroegeneria mtDNA.[7]
Many genera and species of Triticeae are allopolyploids, having more chromosomes than seen in typical diploids. Typical allopolyploids are tetraploid or hexaploid, XXYY or XXYYZZ. The creation of polyploid species results from natural random events tolerated by polyploid-capable plants. Natural allopolyploid plants may have selective advantages and some may permit the recombination of distantly related genetic material. Poulard wheat is an example of a stable allotetraploid wheat.
The Secale (domesticated rye) may be a very early branch from the goat grass clad (or goat grasses are a branch of early rye grasses), as branch these are almost contemporary with the branching between monoploid wheat and Aegilops tauschii. Studies in Anatolia now suggest Rye (Secale) was cultivated, but not domesticated, prior to the holocene and to evidence for the cultivation of wheat. As climate changed the favorablitiy of Secale declined. At that time other strains of barley and wheat may have been cultivated, but humans did little to change them.
Goat grasses and the evolution of bread wheat
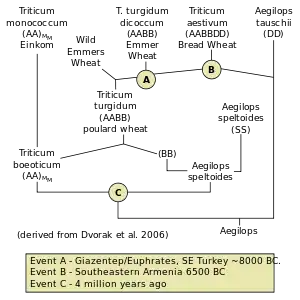
Tetraploidization in wild emmer wheat
Aegilops appears to be basal to several taxa such as Triticum, Amblyopyrum, and Crithopsis. Certain species such as Aegilops speltoides could potentially represent core variants of the taxa. The generic placement may be more a matter of nomenclature. Genera Aegilops and Triticum are very closely related; as the adjacent image illustrates, the Aegilops species occupy most of the basal branch points in bread wheat evolution indicating that genus Triticum evolved from Aegilops after an estimated 4 million years ago.[8] The divergence of the genomes is followed by allotetraploidization of a speltoid goatgrass x basal wheat species Triticum boeoticum with strains in the middle eastern region giving rise to cultivated emmer wheat.[9]
Hexaploidization of tetraploid wheat
Hybridization of tetraploid wheat with Ae. tauschii produced a hulled wheat similar to spelt, suggesting T. spelta is basal. The tauschii species can be subdivided into subspecies tauschii (eastern Turkey to China or Pakistan) and strangulata (Caucasus to S. Caspian, N. Iran). The D genome of bread wheat is closer to A.t. strangulata than A.t. tauschii. It is suggested that Ae. tauschii underwent rapid selective evolution prior to combining with tetraploid wheat.
Wild Triticeae use by humans
Intense use of wild Triticeae can be seen in the Levant as early as 23,000 years ago.[10] This site, Ohala II (Israel), also shows that Triticeae grains were processed and cooked.[11] Many cultivars appear to have been domesticated in the region of the upper Fertile Crescent, Levant and central Anatolia.[12][13] More recent evidence suggests that cultivation of wheat from emmer's wheat required a longer period with wild seeding maintaining a presence in archaeological finds.[14]
Pastoral grasses
Triticeae has a pastoral component that some contend goes back to the Neolithic period and is referred to as the Garden Hunting Hypothesis. In this hypothesis grains could be planted or shared for the purpose of attracting game animals so that they could be hunted close to settlements.
Today, rye and other Triticeae cultivars are used to graze animals, particularly cattle. Rye grasses in the New World have been used selectively as fodder, but also to protect grasslands without the introduction of invasive Old World species.
Triticeae and health
Glutens (storage proteins) in the Triticeae tribe have been linked to gluten-sensitive diseases. While it was once believed that oats carried similar potentials, recent studies indicate that most oat sensitivity is the result of contamination. Triticeae glutens studies are important in determining the links between gluten and gastrointestinal, allergic, and autoimmune diseases.[15] Some of the recently discovered biochemical and immunochemical properties of these proteins suggest they evolved for protection against dedicated or continuous consumption by mammalian seed-eaters.[16][17] One recent publication even raises doubts about wheat's safety for anyone to eat.[18] Overlapping properties with regard to food preparation have made these proteins much more useful as cereal cultivars, and a balanced perspective suggests a variable tolerance to Triticeae glutens reflects early childhood environment and genetic predisposition.[19][20][21][22]
References
- ↑ Soreng, Robert J.; Peterson, Paul M.; Romaschenko, Konstantin; Davidse, Gerrit; Teisher, Jordan K.; Clark, Lynn G.; Barberá, Patricia; Gillespie, Lynn J.; Zuloaga, Fernando O. (2017). "A worldwide phylogenetic classification of the Poaceae (Gramineae) II: An update and a comparison of two 2015 classifications". Journal of Systematics and Evolution. 55 (4): 259–290. doi:10.1111/jse.12262. hdl:10261/240149. ISSN 1674-4918.
- ↑ Eadie M (2004). "Ergot of rye-the first specific for migraine". J Clin Neurosci. 11 (1): 4–7. doi:10.1016/j.jocn.2003.05.002. PMID 14642357. S2CID 45659231.
- ↑ Oliver, R.E.; Cai, X.; Friesen, T.L.; Halley, S.; Stack, R.W.; Xu, S.S. (2008). "Evaluation of Fusarium Head Blight Resistance in Tetraploid Wheat (Triticum turgidum L.)". Crop Science. 48 (1): 213–222. doi:10.2135/cropsci2007.03.0129.
- ↑ Kubo N, Salomon B, Komatsuda T, von Bothmer R, Kadowaki K (2005). "Structural and distributional variation of mitochondrial rps2 genes in the tribe Triticeae (Poaceae)". Theor Appl Genet. 110 (6): 995–1002. doi:10.1007/s00122-004-1839-x. PMID 15754209. S2CID 20620721.
- ↑ Mason-Gamer R (2004). "Reticulate evolution, introgression, and intertribal gene capture in an allohexaploid grass". Syst Biol. 53 (1): 25–37. doi:10.1080/10635150490424402. PMID 14965898.
- ↑ Liu Q, Ge S, Tang H, Zhang X, Zhu G, Lu B (2006). "Phylogenetic relationships in Elymus (Poaceae: Triticeae) based on the nuclear ribosomal internal transcribed spacer and chloroplast trnL-F sequences". New Phytol. 170 (2): 411–20. doi:10.1111/j.1469-8137.2006.01665.x. PMID 16608465.
- ↑ Mason-Gamer R, Orme N, Anderson C (2002). "Phylogenetic analysis of North American Elymus and the monogenomic Triticeae (Poaceae) using three chloroplast DNA data sets". Genome. 45 (6): 991–1002. doi:10.1139/g02-065. PMID 12502243.
- ↑ Dvorak J, Akhunov ED, Akhunov AR, Deal KR, Luo MC (2006). "Molecular characterization of a diagnostic DNA marker for domesticated tetraploid wheat provides evidence for gene flow from wild tetraploid wheat to hexaploid wheat". Mol Biol Evol. 23 (7): 1386–1396. doi:10.1093/molbev/msl004. PMID 16675504.
- ↑ Heun M, Schäfer-Pregl R, Klawan D, Castagna R, Accerbi M, Borghi B, Salamini F (1997). "Site of Einkorn Wheat Domestication Identified by DNA Fingerprinting". Science. 278 (5341): 1312–1314. Bibcode:1997Sci...278.1312H. doi:10.1126/science.278.5341.1312.
- ↑ Weiss E, Wetterstrom W, Nadel D, Bar-Yosef O (2004). "The broad spectrum revisited: Evidence from plant remains". Proc Natl Acad Sci USA. 101 (26): 9551–5. Bibcode:2004PNAS..101.9551W. doi:10.1073/pnas.0402362101. PMC 470712. PMID 15210984.
- ↑ Piperno D, Weiss E, Holst I, Nadel D (2004). "Processing of wild cereal grains in the Upper Palaeolithic revealed by starch grain analysis". Nature. 430 (7000): 670–3. Bibcode:2004Natur.430..670P. doi:10.1038/nature02734. PMID 15295598. S2CID 4431395.
- ↑ Lev-Yadun S, Gopher A, Abbo S (2000). "The Cradle of Agriculture". Science. 288 (5471): 1602–1603. doi:10.1126/science.288.5471.1602. PMID 10858140. S2CID 86661579.
- ↑ Weiss E, Kislev ME, Hartmann A (2006). "(Perspectives-Anthropology:) Autonomous Cultivation Before Domestication". Science. 312 (5780): 1608–1610. doi:10.1126/science.1127235. PMID 16778044. S2CID 83125044.
- ↑ Balter M (2007). "Seeking Agriculture's Ancient Roots". Science. 316 (5833): 1830–1835. doi:10.1126/science.316.5833.1830. PMID 17600193. S2CID 128452153.
- ↑ Silano M, Dessì M, De Vincenzi M, Cornell H (2007). "In vitro tests indicate that certain varieties of oats may be harmful to patients with coeliac disease". J. Gastroenterol. Hepatol. 22 (4): 528–31. doi:10.1111/j.1440-1746.2006.04512.x. PMID 17376046. S2CID 38754601.
- ↑ Mamone G, Ferranti P, Rossi M, et al. (2007). "Identification of a peptide from alpha-gliadin resistant to digestive enzymes: Implications for celiac disease". Journal of Chromatography B. 855 (2): 236–41. doi:10.1016/j.jchromb.2007.05.009. PMID 17544966.
- ↑ Shan L, Qiao SW, Arentz-Hansen H, et al. (2005). "Identification and Analysis of Multivalent Proteolytically Resistant Peptides from Gluten: Implications for Celiac Sprue". J. Proteome Res. 4 (5): 1732–41. doi:10.1021/pr050173t. PMC 1343496. PMID 16212427.
- ↑ Bernardo D, Garrote JA, Fernández-Salazar L, Riestra S, Arranz E (2007). "Is gliadin really safe for non‐coeliac individuals? Production of interleukin 15 in biopsy culture from non‐coeliac individuals challenged with gliadin peptides". Gut. 56 (6): 889–90. doi:10.1136/gut.2006.118265. PMC 1954879. PMID 17519496.
- ↑ Collin P, Mäki M, Kaukinen K (2007). "Safe gluten threshold for patients with celiac disease: some patients are more tolerant than others". Am. J. Clin. Nutr. 86 (1): 260, author reply 260–1. doi:10.1093/ajcn/86.1.260. PMID 17616789.
- ↑ Guandalini S (2007). "The influence of gluten: weaning recommendations for healthy children and children at risk for celiac disease". Issues in Complementary Feeding. Nestlé Nutrition Workshop Series: Pediatric Program. Vol. 60. pp. 139–55. doi:10.1159/000106366. ISBN 978-3-8055-8283-4. PMID 17664902.
{{cite book}}:|journal=ignored (help) - ↑ Bao F, Yu L, Babu S, et al. (1999). "One third of HLA DQ2 homozygous patients with type 1 diabetes express celiac disease-associated transglutaminase autoantibodies". J. Autoimmun. 13 (1): 143–8. doi:10.1006/jaut.1999.0303. PMID 10441179.
- ↑ Zubillaga P, Vidales MC, Zubillaga I, Ormaechea V, García-Urkía N, Vitoria JC (2002). "HLA-DQA1 and HLA-DQB1 genetic markers and clinical presentation in celiac disease". J. Pediatr. Gastroenterol. Nutr. 34 (5): 548–54. doi:10.1097/00005176-200205000-00014. PMID 12050583. S2CID 46271326.
External links
- Pubmed:Triticeae
- Database of Edible Seed Plants
- International Center for Agricultural Research in the Dry Areas (ICARDA) - An excellent resource for the ancestral genetics of Triticeae.
- Aegilops (genome) Comparative Classification Table
- Triticum (genome) Comparative Classification Table
- Genomes in Aegilops, Triticum, and Amblyopyrum
- Triticeae germplasm