 | |
| Names | |
|---|---|
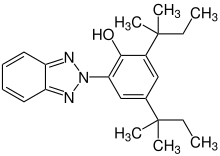
| Preferred IUPAC name
2-(2H-1,2,3-Benzotriazol-2-yl)-4,6-bis(2-methylbutan-2-yl)phenol | |
| Other names
2-(2H-Benzotriazol-2-yl)-4,6-di-tert-pentylphenol; Tinuvin-328; UV-328 | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.043.062 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C22H29N3O | |
| Molar mass | 351.494 g·mol−1 |
| Appearance | Solid |
| Melting point | 80-86 °C[1] |
| 0,17±0,07 μg·l−1 (25 °C)[2] | |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H373, H413 | |
| P260, P273, P314, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
UV-328 (2-(2H-benzotriazol-2-yl)-4,6-di-tert-pentylphenol) is a chemical compound that belongs to the phenolic benzotriazoles. It is a UV filter that is used as an antioxidant for plastics.[3]
Properties
UV-328 has a melting point of 80-86 °C, a vapor pressure of 4,6·10−5 Pa (20 °C) and a water solubility of 0,17±0,07 μg·l−1 (25 °C).[2]
The octanol-water partition coefficient (log KOW) is 7,93.[2][4]
Applications
UV-328 is a light stabilizer for a variety of plastics and other organic substrates. Its use is recommended for the stabilization of styrene homopolymers and copolymers, acrylic polymers, unsaturated polyesters, polyvinyl chloride, polyolefins, polyurethanes, polyacetals, polyvinyl butyral, elastomers and adhesives.[5] It protects polymers and organic pigments from UV radiation and helps maintain the original appearance and physical integrity of moldings, films, sheets and fibers during outdoor weathering. The application concentration is 0.1-1 %.[5]
UV-328 is recommended for applications such as automotive coatings, industrial coatings, commercial inks such as wood stains or do-it-yourself inks.[6]
Hazard
UV-328 is persistent, bioaccumulative and toxic (PBT) as well as very persistent and very bioaccumulative (vPvB). Thus, it is in the list of substances of very high concern.[7] The 2023 Conference of the Parties of the United Nations Stockholm Convention on Persistent Organic Pollutants took the decision to eliminate the use of UV-328, by listing this chemical in Annex A to the Convention.[8]
A bioaccumulation factor (log BAF) of 2,6–3,4 was determined in fish from Canadian rivers.[9] It may cause long lasting harmful effects to aquatic life.[10]
UV-328 has been found to be associated with adverse health effects in mammals based on repeated-dose toxicity studies conducted in rats and dogs, with the primary health effect being liver toxicity. It is also associated with adverse effects on the kidney based on the study in rats.[11]
The finding of UV-328 in plastics sampled on remote beaches,[12] in stomachs of seabirds[13] and in preen gland oil[14][15] show that it is also transported over long distances and is taken up by biota.[16] Detections in Arctic biota include eggs of common eider, kittiwake, European shag and glaucous gull as well as the livers of mink.[17]
References
- ↑ Wypych, Anna (2015-07-08). Databook of UV Stabilizers. Elsevier. ISBN 9781927885031.
- 1 2 3 Anh T. Ngoc Do, Yeonjeong Ha, Hyun-Joong Kang, Ju Min Kim, Jung-Hwan Kwon (April 2022), "Equilibrium leaching of selected ultraviolet stabilizers from plastic products", Journal of Hazardous Materials, vol. 427, p. 128144, doi:10.1016/j.jhazmat.2021.128144, PMID 34979390, S2CID 245562420
{{citation}}: CS1 maint: multiple names: authors list (link) - ↑ "2-(2H-Benzotriazol-2-yl)-4,6-di-tert-pentylphenol". www.trc-canada.com. Retrieved 2019-05-28.
- ↑ Anh T. Ngoc Do, Yeonjeong Ha, Hyun-Joong Kang, Ju Min Kim, Jung-Hwan Kwon (May 2022), "Corrigendum to "Equilibrium leaching of selected ultraviolet stabilizers from plastic products" [J. Hazard. Mater. 427 (2022) 128144]", Journal of Hazardous Materials, vol. 430, Table S1, doi:10.1016/j.jhazmat.2022.128487, PMID 35183055, S2CID 246940514
{{citation}}: CS1 maint: multiple names: authors list (link) - 1 2 "Ciba® TINUVIN® 328 Benzotriazole UV Absorber" (PDF).
- ↑ "Ciba® TINUVIN® 328 Light Stabiliser" (PDF). Ciba Speciality Chemicals.
- ↑ "Candidate List of substances of very high concern for Authorisation - ECHA". echa.europa.eu. Retrieved 2019-05-28.
- ↑ "Governments accelerate action and take bold decisions to address pollution from chemicals and wastes". Secretariat of the Basel, Rotterdam and Stockholm Conventions. May 15, 2023. Retrieved 6 July 2023.
- ↑ Abigaëlle Dalpé Castilloux, Magali Houde, Andrée Gendron, Amila De Silva, Youssouf Djibril Soubaneh, Zhe Lu (2022-04-19), "Distribution and Fate of Ultraviolet Absorbents and Industrial Antioxidants in the St. Lawrence River, Quebec, Canada", Environmental Science & Technology, vol. 56, no. 8, pp. 5009–5019, Bibcode:2022EnST...56.5009C, doi:10.1021/acs.est.1c07932, PMC 9022226, PMID 35395156
{{citation}}: CS1 maint: multiple names: authors list (link) - ↑ "Classifications - CL Inventory". echa.europa.eu. Retrieved 2020-12-24.
- ↑ https://echa.europa.eu/documents/10162/17233/rac_opinion_uv-320-328_en.pdf/34ab1637-2db8-456c-b19c-918db4c99395
- ↑ Tanaka, Kosuke; Takada, Hideshige; Ikenaka, Yoshinori; Nakayama, Shouta M. M.; Ishizuka, Mayumi (2020-01-01). "Occurrence and concentrations of chemical additives in plastic fragments on a beach on the island of Kauai, Hawaii". Marine Pollution Bulletin. 150: 110732. Bibcode:2020MarPB.15010732T. doi:10.1016/j.marpolbul.2019.110732. ISSN 0025-326X. PMID 31757391. S2CID 208233759.
- ↑ Tanaka, Kosuke; van Franeker, Jan A.; Deguchi, Tomohiro; Takada, Hideshige (August 2019). "Piece-by-piece analysis of additives and manufacturing byproducts in plastics ingested by seabirds: Implication for risk of exposure to seabirds". Marine Pollution Bulletin. 145: 36–41. Bibcode:2019MarPB.145...36T. doi:10.1016/j.marpolbul.2019.05.028. ISSN 1879-3363. PMID 31590798. S2CID 181809194.
- ↑ Tanaka, Kosuke; Watanuki, Yutaka; Takada, Hideshige; Ishizuka, Mayumi; Yamashita, Rei; Kazama, Mami; Hiki, Nagako; Kashiwada, Fumika; Mizukawa, Kaoruko; Mizukawa, Hazuki; Hyrenbach, David (February 2020). "In Vivo Accumulation of Plastic-Derived Chemicals into Seabird Tissues". Current Biology. 30 (4): 723–728.e3. doi:10.1016/j.cub.2019.12.037. ISSN 0960-9822. PMID 32008901. S2CID 210957031.
- ↑ Yamashita, Rei; Hiki, Nagako; Kashiwada, Fumika; Takada, Hideshige; Mizukawa, Kaoruko; Hardesty, Britta Denise; Roman, Lauren; Hyrenbach, David; Ryan, Peter G.; Dilley, Ben J.; Muñoz-Pérez, Juan Pablo (2021). "Plastic additives and legacy persistent organic pollutants in the preen gland oil of seabirds sampled across the globe". Environmental Monitoring and Contaminants Research. 1: 97–112. doi:10.5985/emcr.20210009. S2CID 241803983.
- ↑ Andrade, Helena; Glüge, Juliane; Herzke, Dorte; Ashta, Narain Maharaj; Nayagar, Shwetha Manohar; Scheringer, Martin (2021-07-22). "Oceanic long-range transport of organic additives present in plastic products: an overview". Environmental Sciences Europe. 33 (1): 85. doi:10.1186/s12302-021-00522-x. hdl:11250/2767248. ISSN 2190-4715. S2CID 236163411.
- ↑ Schlabach; et al. (2019). "Screening program 2018: Volatiles, Gd, BADGE, UV filters, Additives, and Medicines" (PDF). NILU, NIVA.