 | |
| Names | |
|---|---|
| IUPAC name
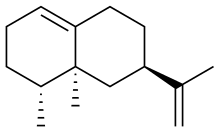
4α,5α-Eremophila-1(10),11-diene | |
| Systematic IUPAC name
(3R,4aS,5R)-4a,5-Dimethyl-3-(prop-1-en-2-yl)-1,2,3,4,4a,5,6,7-octahydronaphthalene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.022.770 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H24 | |
| Molar mass | 204.357 g·mol−1 |
| Boiling point | 123 °C (253 °F; 396 K) at 11 mmHg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Valencene is a sesquiterpene that is an aroma component of citrus fruit and citrus-derived odorants. It is obtained inexpensively from Valencia oranges.[1] Valencene is biosynthesized from farnesyl pyrophosphate (FPP) by the CVS enzyme.
It is a precursor to nootkatone, the main contributor to the aroma and flavor of grapefruit[2] and is often used in insecticides, cleaning, personal care products and cosmetics.[3]
References
- ↑ Furusawa, Mai; Toshihiro Hashimoto; Yoshiaki Noma; Yoshinori Asakawa (November 2005). "Highly Efficient Production of Nootkatone, the Grapefruit Aroma from Valencene, by Biotransformation". Chem. Pharm. Bull. 53 (11): 1513–1514. doi:10.1248/cpb.53.1513. PMID 16272746.
- ↑ M. M. Bomgardner (July 16, 2012). "Fragrances 101. A Fortuitous Field of Flavors and Fragrances". Chemical & Engineering News. 90 (29).
- ↑ "Everything You Need to Know About Valencene and Its Benefits". Trulieve.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.