 | |
| Names | |
|---|---|
| IUPAC name
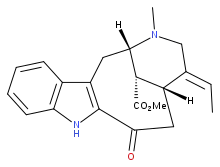
Methyl (1S,14R,15E,18S)-15-ethylidene-17-methyl-12-oxo-10,17-diazatetracyclo[12.3.1.03,11.04,9]octadeca-3(11),4,6,8-tetraene-18-carboxylate | |
| Preferred IUPAC name
Methyl (19E)-3-oxovobasan-17-oate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| |
| |
| Properties | |
| C21H24N2O3 | |
| Molar mass | 352.434 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Vobasine is a naturally occurring monoterpene indole alkaloid found in several species in the genus Tabernaemontana including Tabernaemontana divaricata.[1]
History
Vobasine was first reported by Renner in 1959 after its isolation from Voacanga africana.[2] The two structurally related compounds, dregamine and tabernaemontanine, where its alkene (=CHCH3) sidechain was reduced to ethyl groups in two configurations, had their relationship confirmed in the 1970s.[3][4][5] Vobasine has been found in many plants of the dogbane (Apocynaceae) family including Tabernaemontana dichotoma.[6][7]
Synthesis
Biosynthesis
As with other Indole alkaloids, the biosynthesis of vobasine starts from the amino acid tryptophan. This is converted into strictosidine before further elaboration.[8]
Chemical synthesis
The synthesis of alkaloids with the same carbon skeleton as vobasine began in the 1960s[9] and has continued, with some work providing enantiospecific approaches to closely related compounds.[10]
Natural occurrence

Vobasine is found commonly in the genera Tabernaemontana and Voacanga, including the species Ervatamia hirta,[11] Tabernaemontana elegans,[12] Tabernaemontana divaricata[13][14] and Voacanga africana.[2]
Biochemistry
Plant metabolites have been of interest for their possible biological activity and alkaloids in particular are major subjects for ethnobotanical research.[15][16] Vobasine has been studied, for example as a potential anti-cancer agent[17] and for its hypotensive activity.[18] However, the alkaloid itself has not been developed as a drug.
Toxicity
Very high dose of vobasine at around 300 mg/kg may cause death through CNS and respiratory depression.[19]
See also
References
- ↑ Saxton, J. E. (1987). "Recent progress in the chemistry of indole alkaloids and mould metabolites". Natural Product Reports. 4: 591. doi:10.1039/NP9870400591.
- 1 2 Renner, U. (1959). "Vobasin und Voacryptin, zwei neue Alkaloide aus Voacanga africana Stapf". Experientia. 15 (5): 185–186. doi:10.1007/BF02158691. S2CID 28675532.
- ↑ Renner, U.; Prins, D. A. (1961). "Voacanga-Alkaloide V. Verknüpfung von Vobasin mit Dregamin und Tabernaemontanin". Experientia. 17 (5): 209. doi:10.1007/BF02160617. PMID 13740864. S2CID 35816536.
- ↑ Knox, JR; Slobbe, J. (1975). "Indole alkaloids from Ervatamia orientalis. III. The configurations of the ethyl side chains of dregamine and tabernaemontanine and some further chemistry of the vobasine group". Australian Journal of Chemistry. 28 (8): 1843. doi:10.1071/CH9751843.
- ↑ Bombardelli, Ezio; Bonati, Attilio; Gabetta, Bruno; Martinelli, Ernesto M.; Mustich, Giuseppe; Danieli, Bruno (1976). "Structures of tabernaelegantines A–D and tabernaelegantinines a and B, new indole alkaloids from Tabernaemontana elegans". Journal of the Chemical Society, Perkin Transactions 1 (13): 1432–1438. doi:10.1039/P19760001432.
- ↑ Raffauf, Robert F.; Flagler, M. B. (1960). "Alkaloids of the Apocynaceae". Economic Botany. 14: 37–55. doi:10.1007/BF02859365. S2CID 29538706.
- ↑ Perera, Premila; Samuelsson, Gunnar; Van Beek, Teris; Verpoorte, Robert (1983). "Tertiary Indole Alkaloids from Leaves of Tabernaemontana dichotoma". Planta Medica. 47 (3): 148–150. doi:10.1055/s-2007-969974. PMID 17404903.
- ↑ Edwin Saxton, J. (15 September 2009). Indoles, Part 4: The Monoterpenoid Indole Alkaloids. ISBN 9780470188446.
- ↑ Shioiri, T.; Yamada, S. (1968). "Studies in the indole series—IV". Tetrahedron. 24 (11): 4159–4175. doi:10.1016/0040-4020(68)88178-5. PMID 5654925.
- ↑ Yang, Jie; Rallapalli, Sundari K.; Cook, James M. (2010). "The first enantiospecific total synthesis of the 3-oxygenated sarpagine indole alkaloids affinine and 16-epiaffinine, as well as vobasinediol and 16-epivobasinediol". Tetrahedron Letters. 51 (5): 815–817. doi:10.1016/j.tetlet.2009.12.002.
- ↑ Clivio, Pascale; Richard, Bernard; Deverre, Jean-Robert; Sevenet, Thierry; Zeches, Monique; Le Men-Oliver, Louisette (January 1991). "Alkaloids from leaves and root bark of Ervatamia hirta". Phytochemistry. 30 (11): 3785–3792. doi:10.1016/0031-9422(91)80111-D.
- ↑ Van Der Heijden, R.; Brouwer, R.L.; Verpoorte, R.; Wijnsma, R.; Van Beek, T.A.; Harkes, A.A.; Svendsen, A.Baerheim (1986). "Indole alkaloids from a callus culture of Tabernaemontana elegans". Phytochemistry. 25 (4): 843–846. doi:10.1016/0031-9422(86)80013-9.
- ↑ Kam, Toh-Seok; Pang, Huey-Shen; Lim, Tuck-Meng (2003). "Biologically active indole and bisindole alkaloids from Tabernaemontana divaricata". Organic & Biomolecular Chemistry. 1 (8): 1292–1297. doi:10.1039/B301167D. PMID 12929658.
- ↑ Kulshreshtha, Ankita; Saxena, Jyoti (2019). "Alkaloids and Non Alkaloids of Tabernaemontana divaricata" (PDF). International Journal of Research and Review. 6 (8): 517–524.
- ↑ Pratchayasakul W, Pongchaidecha A, Chattipakorn N, Chattipakorn S (April 2008). "Ethnobotany & ethnopharmacology of Tabernaemontana divaricata" (PDF). The Indian Journal of Medical Research. 127 (4): 317–35. PMID 18577786.
- ↑ Babiaka, Smith B.; Ntie-Kang, Fidele; Lifongo, Lydia L.; Ndingkokhar, Bakoh; Mbah, James A.; Yong, Joseph N. (2015). "The chemistry and bioactivity of Southern African flora I: A bioactivity versus ethnobotanical survey of alkaloid and terpenoid classes". RSC Advances. 5 (54): 43242–43267. Bibcode:2015RSCAd...543242B. doi:10.1039/C5RA01912E.
- ↑ Ferreira, Maria-José U.; Paterna, Angela (2019). "Monoterpene indole alkaloids as leads for targeting multidrug resistant cancer cells from the African medicinal plant Tabernaemontana elegans". Phytochemistry Reviews. 18 (4): 971–987. doi:10.1007/s11101-019-09615-1. S2CID 184483520.
- ↑ Perera, Premila; Kanjanapothy, Duangta; Sandberg, Finn; Verpoorte, Robert (1985). "Muscle relaxant activity and hypotensive activity of some tabernaemontana alkaloids". Journal of Ethnopharmacology. 13 (2): 165–173. doi:10.1016/0378-8741(85)90004-2. PMID 4021514.
- ↑ "Ethnobotany and ethnopharmacology of Tabernaemontana divaricata".
Further reading
- Namjoshi, Ojas A.; Cook, James M. (2016). Sarpagine and Related Alkaloids. The Alkaloids: Chemistry and Biology. Vol. 76. pp. 63–169. doi:10.1016/bs.alkal.2015.08.002. ISBN 9780128046821. PMC 4864735. PMID 26827883.
- Kitajima, Mariko; Takayama, Hiromitsu (2016). Monoterpenoid Bisindole Alkaloids. The Alkaloids: Chemistry and Biology. Vol. 76. pp. 259–310. doi:10.1016/bs.alkal.2015.09.001. ISBN 9780128046821. PMID 26827885.