 | |
| Names | |
|---|---|
| IUPAC name
Antimony(III) sulfate | |
| Other names
Antimonous sulfate Antimony trisulfate Diantimony trisulfate Diantimony tris(sulphate) | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.028.370 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties[1] | |
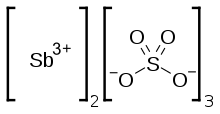
| Sb2(SO4)3 | |
| Molar mass | 531.7078 g/mol |
| Density | 3.6246 g/cm3[2] |
| soluble | |
| Hazards | |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 0.5 mg/m3 (as Sb)[3] |
REL (Recommended) |
TWA 0.5 mg/m3 (as Sb)[3] |
| Safety data sheet (SDS) | MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Antimony sulfate, Sb2(SO4)3, is a hygroscopic salt formed by reacting antimony or its compounds with hot sulfuric acid. It is used in doping of semiconductors and in the production of explosives and fireworks.[2]
Structure
Solid antimony(III) sulfate has a three-dimensional structure: SbO6 octahedra share corners with SO4 tetrahedra.[4]
Chemical properties
Antimony sulfate is deliquescent, and soluble in acids. It can be prepared by dissolving antimony, antimony trioxide, antimony trisulfide or antimony oxychloride in hot, concentrated sulfuric acid.[2][5]
- 2 Sb (s) + 6 H2SO4 → Sb2(SO4)3 + 3 SO2 + 6 H2O
Uses
Owing to its solubility, antimony sulfate has uses in the doping of semiconductors.[6] It is also used for coating anodes in electrolysis and in the production of explosives and fireworks.[2]
Safety
Antimony(III) sulfate causes irritation to the skin and mucous membranes.[7]
Natural occurrence
Natural analogue of the exact compound is yet unknown. However, basic hydrated Sb sulfates are known as the minerals klebelsbergite[8][9] and coquandite.[10][9]
References
- ↑ Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. p. 4.64. ISBN 0-8493-0486-5.
- 1 2 3 4 Herbst, Karl Albert et al. (1985) Antimony and antimony compounds in Ullmann's Encyclopedia of Industrial Chemistry 5th ed., vol. A3, p. 70. ISBN 3-527-20103-3.
- 1 2 NIOSH Pocket Guide to Chemical Hazards. "#0036". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Materials Data on Sb2(SO4)3 by Materials Project
- ↑ Nicholas C. Norman (31 December 1997). Chemistry of arsenic, antimony, and bismuth. Springer. pp. 193–. ISBN 978-0-7514-0389-3.
- ↑ Method of forming phase change layer, method of manufacturing a storage node using the same, and method of manufacturing phase change memory device using the same – Samsung Electronics Co., Ltd. Freepatentsonline.com (2007-01-02). Retrieved on 2011-12-23.
- ↑ Antimony(III) Sulfate Material Safety Data Sheet Archived 2012-04-26 at the Wayback Machine. Prochemonline.
- ↑ "Klebelsbergite".
- 1 2 "List of Minerals". 21 March 2011.
- ↑ "Coquandite".