 | |
 | |
| Names | |
|---|---|
| IUPAC names
Antimony(III) sulfide Diantimony trisulfide | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.014.285 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Sb2S3 | |
| Molar mass | 339.70 g·mol−1 |
| Appearance | Grey or black orthorhombic crystals (stibnite) |
| Density | 4.562g cm−3 (stibnite)[1] |
| Melting point | 550 °C (1,022 °F; 823 K) (stibnite)[1] |
| Boiling point | 1,150 °C (2,100 °F; 1,420 K) |
| 0.00017 g/(100 mL) (18 °C) | |
| −86.0·10−6 cm3/mol | |
Refractive index (nD) |
4.046 |
| Thermochemistry | |
Heat capacity (C) |
123.32 J/(mol·K) |
Std enthalpy of formation (ΔfH⦵298) |
−157.8 kJ/mol |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
> 2000 mg/kg (rat, oral) |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 0.5 mg/m3 (as Sb)[2] |
REL (Recommended) |
TWA 0.5 mg/m3 (as Sb)[2] |
| Related compounds | |
Other anions |
|
Other cations |
Arsenic trisulfide Bismuth(III) sulfide |
Related compounds |
Antimony pentasulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Antimony trisulfide (Sb2S3) is found in nature as the crystalline mineral stibnite and the amorphous red mineral (actually a mineraloid)[3] metastibnite.[4] It is manufactured for use in safety matches, military ammunition, explosives and fireworks. It also is used in the production of ruby-colored glass and in plastics as a flame retardant.[5] Historically the stibnite form was used as a grey pigment in paintings produced in the 16th century.[6] In 1817, the dye and fabric chemist, John Mercer discovered the non-stoichiometric compound Antimony Orange (approximate formula Sb2S3·Sb2O3), the first good orange pigment available for cotton fabric printing.[7]
Antimony trisulfide was also used as the image sensitive photoconductor in vidicon camera tubes. It is a semiconductor with a direct band gap of 1.8–2.5 eV. With suitable doping, p and n type materials can be produced.[8]
Preparation and reactions
Sb2S3 can be prepared from the elements at temperature 500–900 °C:[5]
- 2 Sb + 3 S → Sb2S3
Sb2S3 is precipitated when H2S is passed through an acidified solution of Sb(III).[9] This reaction has been used as a gravimetric method for determining antimony, bubbling H2S through a solution of Sb(III) compound in hot HCl deposits an orange form of Sb2S3 which turns black under the reaction conditions.[10]
Sb2S3 is readily oxidised, reacting vigorously with oxidising agents.[5] It burns in air with a blue flame. It reacts with incandescence with cadmium, magnesium and zinc chlorates. Mixtures of Sb2S3 and chlorates may explode.[11]
In the extraction of antimony from antimony ores the alkaline sulfide process is employed where Sb2S3 reacts to form thioantimonate(III) salts (also called thioantimonite):[12]
- 3 Na2S + Sb2S3 → 2 Na3SbS3
A number of salts containing different thioantimonate(III) ions can be prepared from Sb2S3. These include:[13]
- [SbS3]3−, [SbS2]−, [Sb2S5]4−, [Sb4S9]6−, [Sb4S7]2− and [Sb8S17]10−
Schlippe's salt, Na3SbS4·9H2O, a thioantimonate(V) salt is formed when Sb2S3 is boiled with sulfur and sodium hydroxide. The reaction can be represented as:[9]
- Sb2S3 + 3 S2− + 2 S → 2 [SbS4]3−
Structure
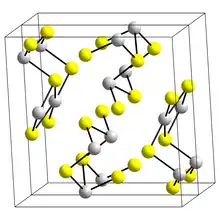
The structure of the black needle-like form of Sb2S3, stibnite, consists of linked ribbons in which antimony atoms are in two different coordination environments, trigonal pyramidal and square pyramidal.[9] Similar ribbons occur in Bi2S3 and Sb2Se3.[14] The red form, metastibnite, is amorphous. Recent work suggests that there are a number of closely related temperature dependent structures of stibnite which have been termed stibnite (I) the high temperature form, identified previously, stibnite (II) and stibnite (III).[15] Other paper shows that the actual coordination polyhedra of antimony are in fact SbS7, with (3+4) coordination at the M1 site and (5+2) at the M2 site. These coordinations consider the presence of secondary bonds. Some of the secondary bonds impart cohesion and are connected with packing.[16]
References
- 1 2 Haynes, W. M., ed. (2014). CRC Handbook of Chemistry and Physics (95th ed.). Boca Raton, FL: CRC Press. pp. 4–48. ISBN 978-1-4822-0867-2.
- 1 2 NIOSH Pocket Guide to Chemical Hazards. "#0036". National Institute for Occupational Safety and Health (NIOSH).
- ↑ "Metastibnite".
- ↑ SUPERGENE METASTIBNITE FROM MINA ALACRAN, PAMPA LARGA, COPIAPO, CHILE, Alan H Clark, THE AMERICAN MINERALOGIST. VOL. 55., 1970
- 1 2 3 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 581–582. ISBN 978-0-08-037941-8.
- ↑ Eastaugh, Nicholas (2004). Pigment Compendium: A Dictionary of Historical Pigments. Butterworth-Heinemann. p. 359. ISBN 978-0-7506-5749-5.
- ↑ Parnell, Edward A (1886). The life and labours of John Mercer. London: Longmans, Green & Co. p. 23.
- ↑ Electrochemistry of Metal Chalcogenides, Mirtat Bouroushian, Springer, 2010
- 1 2 3 Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, p. 765-766, ISBN 0-12-352651-5
- ↑ A.I. Vogel, (1951), Quantitative Inorganic analysis, (2d edition), Longmans Green and Co
- ↑ Hazardous Laboratory Chemicals Disposal Guide, Third Edition, CRC Press, 2003, Margaret-Ann Armour, ISBN 9781566705677
- ↑ Anderson, Corby G. (2012). "The metallurgy of antimony". Chemie der Erde - Geochemistry. 72: 3–8. Bibcode:2012ChEG...72....3A. doi:10.1016/j.chemer.2012.04.001. ISSN 0009-2819.
- ↑ Inorganic Reactions and Methods, The Formation of Bonds to Group VIB (O, S, Se, Te, Po) Elements (Part 1) (Volume 5) Ed. A.P, Hagen,1991, Wiley-VCH, ISBN 0-471-18658-9
- ↑ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- ↑ Kuze S., Du Boulay D., Ishizawa N., Saiki A, Pring A.; (2004), X ray diffraction evidence for a monoclinic form of stibnite, Sb2S3, below 290K; American Mineralogist, 9(89), 1022-1025.
- ↑ Kyono, A.; Kimata, M.; Matsuhisa, M.; Miyashita, Y.; Okamoto, K. (2002). "Low-temperature crystal structures of stibnite implying orbital overlap of Sb 5s 2 inert pair electrons". Physics and Chemistry of Minerals. 29 (4): 254–260. Bibcode:2002PCM....29..254K. doi:10.1007/s00269-001-0227-1. S2CID 95067785.
