 | |
| Names | |
|---|---|
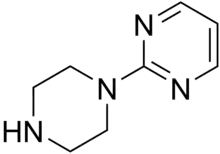
| Preferred IUPAC name
2-(Piperazin-1-yl)pyrimidine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.040.107 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H12N4 | |
| Molar mass | 164.21 g/mol |
| Hazards | |
| GHS labelling: | |
  | |
| Danger | |
| H314, H315, H319, H335 | |
| P260, P261, P264, P271, P280, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P321, P332+P313, P337+P313, P362, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
1-(2-Pyrimidinyl)piperazine (1-PP, 1-PmP) is a chemical compound and piperazine derivative. It is known to act as an antagonist of the α2-adrenergic receptor (Ki = 7.3–40 nM)[1] and, to a much lesser extent, as a partial agonist of the 5-HT1A receptor (Ki = 414 nM; Emax = 54%).[2][3] It has negligible affinity for the dopamine D2, D3, and D4 receptors (Ki > 10,000 nM) and does not appear to have significant affinity for the α1-adrenergic receptors.[4] Its crystal structure has been determined.[5]
Derivatives
A number of pyrimidinylpiperazine derivatives are drugs, including:
- Buspirone – anxiolytic
- Dasatinib – anticancer agent
- Eptapirone – anxiolytic
- Gepirone – anxiolytic
- Ipsapirone – anxiolytic
- Piribedil – antiparkinsonian agent
- Revospirone – anxiolytic
- Tandospirone – anxiolytic
- Tirilazad – neuroprotective agent
- Umespirone – anxiolytic
- Zalospirone – anxiolytic
The anxiolytics are also classified as azapirones due to the azaspirodecanedione moiety in their structures. 1-PP is a common metabolite of most or all of the listed agents.[1][6] Alnespirone, binospirone, and enilospirone, despite being azapirones, are not piperazines and therefore do not metabolize to 1-PP, and while perospirone and tiospirone are piperazines, they are instead benzothiazole-substituted piperazines and do not metabolize to 1-PP either.
See also
References
- 1 2 Blier P, Curet O, Chaput Y, de Montigny C (1991). "Tandospirone and its metabolite, 1-(2-pyrimidinyl)-piperazine--II. Effects of acute administration of 1-PP and long-term administration of tandospirone on noradrenergic neurotransmission". Neuropharmacology. 30 (7): 691–701. doi:10.1016/0028-3908(91)90176-c. PMID 1681447. S2CID 44297577.
- ↑ Zuideveld KP, Rusiç-Pavletiç J, Maas HJ, Peletier LA, Van der Graaf PH, Danhof M (2002). "Pharmacokinetic-pharmacodynamic modeling of buspirone and its metabolite 1-(2-pyrimidinyl)-piperazine in rats". J. Pharmacol. Exp. Ther. 303 (3): 1130–7. doi:10.1124/jpet.102.036798. PMID 12438536. S2CID 14139919.
- ↑ Gobert, A.; Newman-Tancredi, A.; Rivet, J.M.; Audinot, V.; Millan, M.J. (1997). "P.1.047 Yohimbine is a potent, partial agonist at rat and cloned, human serotonin1A receptors: A comparison to buspirone and its metabolite, 1-pyrimidinylpiperazine". European Neuropsychopharmacology. 7: S149–S150. doi:10.1016/S0924-977X(97)88496-9. ISSN 0924-977X. S2CID 54355225.
- ↑ Bergman J, Roof RA, Furman CA, Conroy JL, Mello NK, Sibley DR, Skolnick P (2013). "Modification of cocaine self-administration by buspirone (buspar®): potential involvement of D3 and D4 dopamine receptors". Int. J. Neuropsychopharmacol. 16 (2): 445–58. doi:10.1017/S1461145712000661. PMC 5100812. PMID 22827916.
- ↑ Yamuna, T. S.; Jasinski, J. P.; Kaur, M.; Anderson, B. J.; Yathirajan, H. S. (1 October 2014). "Crystal structures of 4-(pyrimidin-2-yl)piperazin-1-ium chloride and 4-(pyrimidin-2-yl)piperazin-1-ium nitrate". Acta Crystallographica Section E: Structure Reports Online. 70 (10): 203–206. doi:10.1107/S1600536814020169. PMC 4257175. PMID 25484652.
- ↑ Astier B, Lambás Señas L, Soulière F, Schmitt P, Urbain N, Rentero N, Bert L, Denoroy L, Renaud B, Lesourd M, Muñoz C, Chouvet G (2003). "In vivo comparison of two 5-HT1A receptors agonists alnespirone (S-20499) and buspirone on locus coeruleus neuronal activity". Eur. J. Pharmacol. 459 (1): 17–26. doi:10.1016/s0014-2999(02)02814-5. PMID 12505530.