 | |
 | |
| Clinical data | |
|---|---|
| Other names | 3-methylfentanyl, Mefentanyl |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
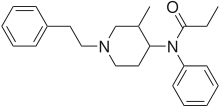
| Formula | C23H30N2O |
| Molar mass | 350.506 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| | |
3-Methylfentanyl (3-MF, mefentanyl) is an opioid analgesic that is an analog of fentanyl. 3-Methylfentanyl is one of the most potent opioids, estimated to be between 400 and 6000 times stronger than morphine,[1] depending on which isomer is used (with the cis isomers being the more potent ones).[2][3]
Overview and history
3-methylfentanyl was first discovered in 1974[4] and subsequently appeared on the street as an alternative to the clandestinely produced fentanyl analog α-methylfentanyl. However, it quickly became apparent that 3-methylfentanyl was much more potent than α-methylfentanyl, and correspondingly more dangerous.[5]

While 3-methylfentanyl was initially sold on the black market for only a short time between 1984 and 1985, its high potency made it an attractive target to clandestine drug producers, as racemic 3-MF is 10–15 times more potent than fentanyl, and so correspondingly larger amounts of cut product for street sales can be produced for an equivalent amount of effort as for producing fentanyl itself; one gram of 3-methylfentanyl might be sufficient to produce several thousand dosage units once diluted for sale. 3-MF has thus reappeared several times, at various places around the world.[6]
The only country in the world with significant (200+ deaths a year, more than 10,000 addicts) abuse of this chemical is Estonia, where a dose of 3-MF costs 10 €, and other opiates are not generally available since the end of the 2000s.[7][8] Approximately 1100 deaths from fentanyl and 3-MF abuse were recorded in Estonia between 2005–2013, compared to approximately 450 deaths in Sweden, Germany, UK, Finland and Greece combined during the same period.[9]
Other opioid analogs even more potent still than 3-MF are known, such as carfentanil and ohmefentanyl,[10] but these are significantly more difficult to manufacture than 3-methylfentanyl. Since 2016 fentanyl seizures in Estonia contains mostly carfentanil or cyclopropylfentanyl.[11]
3-methylfentanyl has similar effects to fentanyl, but is far more potent due to increased binding affinity to its target site. Since fentanyl itself is already highly potent, 3-methylfentanyl is extremely dangerous when used recreationally, and has resulted in many deaths among recreational opioid users ingesting the drug. Side effects of fentanyl analogs are similar to those of fentanyl itself, which include itching, nausea and potentially serious respiratory depression, which can be life-threatening. Fentanyl analogs have killed hundreds of people throughout Europe and the former Soviet republics since the most recent resurgence in use began in Estonia in the early 2000s, and novel derivatives continue to appear.[9]
Use as chemical weapon
3-Methylfentanyl was also reported by media as the identity of the anaesthetic "gas" Kolokol-1 delivered as an aerosol[12] during the Moscow theater hostage crisis in 2002 in which many hostages died from accidental overdoses, 3-methylfentanyl was later ruled out as the primary agent used. The opiate antidote naloxone was on-hand to treat the victims of the crisis, but, whether due to their incarceration, lack of food, or water, or sleep, or due to the novel nature of the still-unconfirmed compound used, acute symptoms continued to develop, resulting in many fatalities despite the administration of naloxone.
Synthesis
A number of methods for synthesis have been published. The most recent is probably the method posted by the Serbian chemical society (2004).[13]
There is another method, though, for constructing the N-Benzyl-3-methyl-4-piperidone in a 2-stage Michael reaction, followed by Dieckmann cyclization as per usual.
See also
References
- ↑ Henderson GL. Designer Drugs: Past History and Future Prospects Journal of Forensic Sciences. 33(2): 569-575 (1988)
- ↑ Jin WQ, Xu H, Zhu YC, Fang SN, Xia XL, Huang ZM, et al. (May 1981). "Studies on synthesis and relationship between analgesic activity and receptor affinity for 3-methyl fentanyl derivatives". Scientia Sinica. 24 (5): 710–20. PMID 6264594.
- ↑ Wang ZX, Zhu YC, Chen XJ, Ji RY (1993). "[Stereoisomers of 3-methylfentanyl: synthesis, absolute configuration and analgesic activity]". Yao Xue Xue Bao = Acta Pharmaceutica Sinica. 28 (12): 905–10. PMID 8030414.
- ↑ Van Bever WF, Niemegeers CJ, Janssen PA (October 1974). "Synthetic analgesics. Synthesis and pharmacology of the diastereoisomers of N-(3-methyl-1-(2-phenylethyl)-4-piperidyl)-N-phenylpropanamide and N-(3-methyl-1-(1-methyl-2-phenylethyl)-4-piperidyl)-N-phenylpropanamide". Journal of Medicinal Chemistry. 17 (10): 1047–51. doi:10.1021/jm00256a003. PMID 4420811.
- ↑ Ayres WA, Starsiak MJ, Sokolay P (1981). "The bogus drug: Three methyl & alpha methyl fentanyl sold as 'China White'". Journal of Psychoactive Drugs. 13 (1): 91–3. doi:10.1080/02791072.1981.10471455. PMID 7277090.
- ↑ Hibbs J, Perper J, Winek CL (February 1991). "An outbreak of designer drug-related deaths in Pennsylvania". JAMA. 265 (8): 1011–3. doi:10.1001/jama.265.8.1011. PMID 1867667.
- ↑ Ojanperä I, Gergov M, Liiv M, Riikoja A, Vuori E (September 2008). "An epidemic of fatal 3-methylfentanyl poisoning in Estonia". International Journal of Legal Medicine. 122 (5): 395–400. doi:10.1007/s00414-008-0230-x. PMID 18386033. S2CID 9626277.
- ↑ "Drug market in Estonia". EMCDDA.
- 1 2 Mounteney J, Giraudon I, Denissov G, Griffiths P (July 2015). "Fentanyls: Are we missing the signs? Highly potent and on the rise in Europe". The International Journal on Drug Policy. 26 (7): 626–31. doi:10.1016/j.drugpo.2015.04.003. PMID 25976511.
- ↑ Huang ZM, Zhou J, Chen XJ, Zheng WJ, Zhang HP, Chi ZQ, et al. (September 1984). "[Analgesic activity and toxicity of potent analgesics, ohmefentanyl and mefentanyl]". Zhongguo Yao Li Xue Bao = Acta Pharmacologica Sinica. 5 (3): 153–8. PMID 6239505.
- ↑ Anneli, Uusküla (2020). "The fentanyl epidemic in Estonia: factors in its evolution and opportunities for a comprehensive public health response, a scoping review". International Journal of Drug Policy. 81: 102757. doi:10.1016/j.drugpo.2020.102757. PMC 7337094. PMID 32416523.
- ↑ "Moscow Theater Crisis: Unknown Chemical Agent Revisited". Bruker Detection. March 10, 2014.
- ↑ Ivanović M, Micovic IV, Vuckovic S, Prostran M, Todorović ZB, Kiricojevic VD, Đorđević J, Došen-Mićović L (2004). "The synthesis and preliminary pharmacological evaluation of the racemic cis and trans 3-alkylfentanyl analogues" (PDF). Journal of the Serbian Chemical Society. 69 (7): 511–26. doi:10.2298/JSC0407511I.