 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.033.455 |
| Chemical and physical data | |
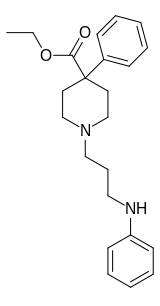
| Formula | C23H30N2O2 |
| Molar mass | 366.505 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Piminodine (Alvodine) is an opioid analgesic that is an analogue of pethidine (meperidine).[2] It was used in medicine briefly during the 1960s and 70s, but has largely fallen out of clinical use. It was used particularly for obstetric analgesia and in dental procedures and, like pethidine, could be combined with hydroxyzine to intensify the effects. The duration of action is 2–4 hours; 7.5–10 mg via the subcutaneous route is the most common starting dose, being equal to 80–100 mg of pethidine, 40–60 mg of alphaprodine and 10 mg of morphine. Oral formulations were also available.
Piminodine has similar effects to other opioids, and produces analgesia, sedation and euphoria. Side effects can include itching, nausea and potentially serious respiratory depression which can be life-threatening.[3][4]
References
- ↑ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ↑ Sim E, Dimoglo A, Shvets N, Ahsen V (August 2002). "Electronic-topological study of the structure-activity relationships in a series of piperidine morphinomimetics". Current Medicinal Chemistry. 9 (16): 1537–45. doi:10.2174/0929867023369510. PMID 12171562.
- ↑ Dekornfeld TJ, Lasagna L (August 1960). "The analgesic potency of piminodine (alvodine)". Journal of Chronic Diseases. 12 (2): 252–7. doi:10.1016/0021-9681(60)90102-8. PMID 13815493.
- ↑ Woods LA, Deneau GA, Bennett DR, Domino EF, Seevers MH (May 1961). "A comparison of the pharmacology of two potent analgesic agents, piminodine (Win 14,098-2) and Win 13,797, with morphine and meperidine". Toxicology and Applied Pharmacology. 3: 358–79. doi:10.1016/0041-008x(61)90072-2. hdl:2027.42/32367. PMID 13786579.