 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
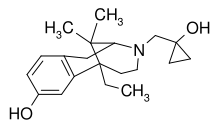
| Formula | C20H29NO2 |
| Molar mass | 315.457 g·mol−1 |
| 3D model (JSmol) | |
| |
Bremazocine is a κ-opioid receptor agonist related to pentazocine. It has potent and long-lasting analgesic and diuretic effects.[1] It has 200 times the activity of morphine, but appears to have no addictive properties and does not depress breathing.[2] The crystal structure of bremazocine was determined in 1984 [3]
See also
References
- ↑ Dortch-Carnes J, Potter DE (2005). "Bremazocine: a kappa-opioid agonist with potent analgesic and other pharmacologic properties". CNS Drug Reviews. 11 (2): 195–212. doi:10.1111/j.1527-3458.2005.tb00270.x. PMC 6741727. PMID 16007240.
- ↑ Patrick G (2013). An Introduction to Medicinal Chemistry (5th expanded ed.). Oxford University Press. p. 641. ISBN 978-0199697397.
- ↑ Verlinde CL, Blaton NM, De Ranter CJ, Peeters OM (1984). "5-Ethyl-2'-hydroxy-2-[(1-hydroxycyclopropyl) methyl]-9, 9-dimethyl-6, 7-benzomorphan hydrochloride (bremazocine), C20H29NO2. HCl". Acta Crystallogr. C. 40 (10): 1759–1761. Bibcode:1984AcCrC..40.1759V. doi:10.1107/S0108270184009434.
| Psychedelics (5-HT2A agonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dissociatives (NMDAR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Deliriants (mAChR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| μ-opioid (MOR) |
| ||||
|---|---|---|---|---|---|
| δ-opioid (DOR) |
| ||||
| κ-opioid (KOR) |
| ||||
| Nociceptin (NOP) |
| ||||
| Others |
| ||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.