 | |
| Clinical data | |
|---|---|
| Other names | 6-acetylcodeine |
| Routes of administration | Intravenous (if present in heroin) |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.169.473 |
| Chemical and physical data | |
| Formula | C20H23NO4 |
| Molar mass | 341.407 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
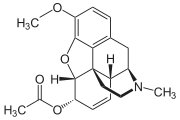
6-Monoacetylcodeine (6-MAC) is an acetate ester of codeine in which the hydroxyl group on the 6 position has been acetylated. It is occasionally present as an impurity in street heroin and is typically created when attempting to create heroin from a solution of morphine in which some of the codeine from the original opium solution still remains. It is formed either through the addition of acetic anhydride, which can only acetylate the 6 position on the codeine or as a result of the addition of acetic acid with a catalyst in an attempt to create 6-monoacetylmorphine, the equivalent ester of morphine which is slightly more potent than heroin itself. 6-monoacetylcodeine is eventually metabolised into codeine and then into morphine. Since only illegally produced heroin is likely to contain 6-MAC, testing for the presence of it in the urine can be used as a fairly reliable method of detecting the use of illicit heroin, as opposed to prescription painkillers.[1] 6-MAC is the precursor for 14-hydroxycodeinenone which was the original precursor to oxycodone. The 7-8 double-bond was reduced using the hyposulfite ion to reduce the 6-7 double-bond.[2] While the acute toxicity of 6-monoacetylcodeine has not been studied in man, animal studies have shown that in animal models its convulsant effects have been proved and when mixed with mono- or di- acetyl morphine, lowers the convulsant threshold of the mixture still further.[3]
See also
- 6-Monoacetylmorphine, the equivalent form of morphine
- Codeine
- Poppy tea
References
- ↑ Staub C, Marset M, Mino A, Mangin P (February 2001). "Detection of acetylcodeine in urine as an indicator of illicit heroin use: method validation and results of a pilot study". Clinical Chemistry. 47 (2): 301–7. doi:10.1093/clinchem/47.2.301. PMID 11159779.
- ↑ Freund M, Speyer E (24 November 1916). "Über die Umwandlung von Thebain in Oxycodeinon und dessen Derivate" [On the conversion of thebaine into oxycodeinone and its derivatives]. Journal für Praktische Chemie (in German). Leipzig. 94 (1): 135–178, 156–157. doi:10.1002/prac.19160940112.
- ↑ O'Neal CL, Poklis A, Lichtman AH (December 2001). "Acetylcodeine, an impurity of illicitly manufactured heroin, elicits convulsions, antinociception, and locomotor stimulation in mice". Drug and Alcohol Dependence. 65 (1): 37–43. doi:10.1016/s0376-8716(01)00145-4. PMID 11714588.