 | |
.png.webp) | |
| Clinical data | |
|---|---|
| Trade names | Minocin, Amzeeq, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682101 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous, topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 90–100%[4] |
| Protein binding | 70–75%[5] |
| Metabolism | Liver[5] |
| Elimination half-life | 14–22[5] (11–26[4]) hours |
| Excretion | Mostly fecal, 10–15% renal[5] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.226.626 |
| Chemical and physical data | |
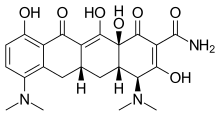
| Formula | C23H27N3O7 |
| Molar mass | 457.483 g·mol−1 |
| 3D model (JSmol) | |
| Specific rotation | = −166°[5] |
| Solubility in water | Low |
| |
| |
| | |
Minocycline, sold under the brand name Minocin among others, is a tetracycline antibiotic medication used to treat a number of bacterial infections such as pneumonia.[2][4][7] It is generally (but not always) less preferred than the tetracycline doxycycline.[4][7] Minocycline is also used for the treatment of acne and rheumatoid arthritis.[7][3] It is taken by mouth or applied to the skin.[4][3]
Common side effects include nausea, diarrhea, dizziness, allergic reactions, and kidney problems.[4] Serious side effects may include anaphylaxis, a lupus-like syndrome, and easy sunburning.[4] Use in the later part of pregnancy may harm the baby and safety during breastfeeding is unclear.[8] It works by decreasing a bacterium's ability to make protein thus stopping its growth.[4]
Minocycline was patented in 1961 and came into commercial use in 1971.[9] It is available as a generic medication.[7][10] In 2020, it was the 236th most commonly prescribed medication in the United States, with more than 1 million prescriptions.[11][12]
Medical uses

Acne
In the United States, minocycline is indicated to treat inflammatory lesions of non-nodular moderate to severe acne vulgaris in people nine years of age and older.[3]
Minocycline and doxycycline are frequently used for the treatment of acne vulgaris.[13][14] Both of these closely related antibiotics have similar levels of efficacy, although doxycycline has a slightly lower risk of adverse side effects.[15] Historically, minocycline has been an effective treatment for acne vulgaris.[16] However, acne that is caused by antibiotic-resistant bacteria is a growing problem in many countries.[17] In Europe and North America, a number of people with acne no longer respond well to treatment with tetracycline family antibiotics because their acne symptoms are caused by bacteria (primarily Cutibacterium acnes) that are resistant to these antibiotics. In order to reduce resistance rates as well as increase the effectiveness of treatment, oral antibiotics should be generally combined with topical acne creams such as benzoyl peroxide or a retinoid (tretinoin, adapalene, etc.).[18]
Infections
Minocycline is also used for other skin infections such as methicillin-resistant Staphylococcus aureus.[19]
Although minocycline's broader spectrum of activity, compared with other members of the group, includes activity against Neisseria meningitidis,[20] its use for prophylaxis is no longer recommended because of side effects (dizziness and vertigo).
It may be used to treat certain strains of methicillin-resistant S. aureus infection and a disease caused by drug-resistant Acinetobacter spp.[21]
A list of uses includes:
- Amoebic dysentery
- Anthrax
- Bubonic plague
- Cholera
- Ehrlichiosis
- Gonorrhea (when penicillin cannot be given)
- Gougerot-Carteaud syndrome (confluent and reticulated papillomatosis)
- Hidradenitis suppurativa
- For use as an adjuvant to HAART[22]
- Leprosy[23]
- Periodontal disease
- Perioral dermatitis[24]
- Respiratory infections such as pneumonia
- Rocky Mountain spotted fever
- Rosacea
- Syphilis (when penicillin cannot be given)
- Urinary tract infections, rectal infections, and infections of the cervix caused by certain microbes
Other
Both minocycline and doxycycline have shown effectiveness in asthma due to immune-suppressing effects.[25] Minocycline and doxycycline have modest effectiveness in treating rheumatoid arthritis.[26] However, the 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis does not include minocycline.[27] Recent research indicate that centrally infused minocycline attenuates brain microglial activation, neuroinflammation and sympathetic activation during pulmonary hypertension.[28]
Contraindications
The drug is contraindicated in people with known hypersensitivity to tetracycline antibiotics, as there is complete cross sensitivity in this group. It is also contraindicated in people with severe liver impairment and after the 16th week of pregnancy.[5]
Side effects
Minocycline may cause upset stomach, diarrhea, dizziness, unsteadiness, drowsiness, mouth sores, headache, and vomiting. It increases sensitivity to sunlight, and may affect the quality of sleep and rarely causes sleep disorders.[29] It has also been linked to cases of lupus.[30] Prolonged use of minocycline can lead to blue-gray staining of skin, fingernails, and scar tissue. This staining is not permanent, but can take a very long time for the skin color to return to normal; however, a muddy brown skin color in sun-exposed areas is usually permanent.[31] Permanent blue discoloration of gums or teeth discoloration may also occur. Rare but serious side effects include fever, yellowing of the eyes or skin, stomach pain, sore throat, vision changes, and mental changes, including depersonalization.[32][33]
Occasionally, minocycline therapy may result in autoimmune disorders such as drug-related lupus and autoimmune hepatitis, which usually occurs in men who also developed minocycline-induced lupus; however, women are more likely to develop minocycline-induced lupus. Significant or complete recovery occurs in most people who develop minocycline-induced autoimmune problems within a period of a few weeks to a year of cessation of minocycline therapy. Autoimmune problems emerge during chronic therapy, but can sometimes occur after only short courses of a couple of weeks of therapy.[34][35] Drug reaction with eosinophilia and systemic symptoms syndrome can occur during the first few weeks of therapy with minocycline.[35]
Minocycline, but not other tetracyclines, can cause vestibular disturbances with dizziness, ataxia, vertigo, and tinnitus. These effects are thought to be related to minocycline's greater penetration into the central nervous system. Vestibular side effects are much more common in women than in men, occurring in 50 to 70% of women receiving minocycline. As a result of the frequency of this bothersome side effect, minocycline is rarely used in female patients.[36] Minocycline's vestibular side effects typically resolve after discontinuation of the drug.[37][38][39]
Symptoms of an allergic reaction include rash, itching, swelling, severe dizziness, and trouble breathing.[32] Minocycline has also been reported to very rarely cause idiopathic intracranial hypertension (pseudotumor cerebri),[40] a side effect also more common in female patients, potentially leading to permanent vision damage if not recognized early and treated.[41]
Contrary to most other tetracycline antibiotics (doxycycline excluded), minocycline may be used in those with kidney disease, but may aggravate systemic lupus erythematosus.[42] It may also trigger or unmask autoimmune hepatitis.[43]
Minocycline can cause the rare condition of secondary intracranial hypertension, which has initial symptoms of headache, visual disturbances, dizziness, vomiting, and confusion.[44] Brain swelling and rheumatoid arthritis are rare side effects of minocycline in some people.[45]
Minocycline, like most tetracyclines, becomes dangerous past its expiration date.[46] While most prescription drugs lose potency after their expiration dates, tetracyclines are known to become toxic over time. Expired tetracyclines can cause serious damage to the kidney due to the formation of a degradation product, anhydro-4-epitetracycline.[46] Minocycline's absorption is impaired if taken at the same time of day as calcium or iron supplements. Unlike some of the other tetracycline group antibiotics, it can be taken with calcium-rich foods such as milk, although this does reduce the absorption slightly.[47]
Minocycline, like other tetracyclines, is associated with esophageal irritation and ulceration if insufficient fluids are taken with the drug before sleep.[48]
A 2007 study suggested that minocycline harms amyotrophic lateral sclerosis patients. Patients on minocycline declined more rapidly than those on placebo. The mechanism of this side effect is unknown, although a hypothesis is that the drug exacerbated an autoimmune component of the primary disease. The effect does not seem to be dose-dependent because the patients on high doses did not do worse than those on the low doses.[49]
The use of minocycline in acne vulgaris has been associated with skin and gut dysbiosis (see antibiotic misuse).[50]
Interactions
The combination of minocycline with dairy, antacids, calcium and magnesium supplements, iron products, laxatives containing magnesium, or bile acid sequestrants may decrease minocycline's effectiveness by forming chelates. Combining it with isotretinoin, acitretin or other retinoids can increase the risk for intracranial hypertension. Minocycline significantly reduces concentrations of the anti-HIV drug atazanavir in the body.[5][51]
Pharmacology
Mechanism of action
Pharmacokinetics
Minocycline is quickly and nearly completely absorbed from the upper part of the small intestine. Taking it together with food, including milk, has no relevant influence on resorption. It reaches highest blood plasma concentrations after one to two hours and has a plasma protein binding of 70–75%. The substance penetrates into almost all tissues; very high concentrations are found in the gallbladder and liver. It crosses the blood–brain barrier better than doxycycline and other tetracyclines, reaching therapeutically relevant concentrations in the cerebrospinal fluid and also in inflamed meninges.[5][52]
Minocycline is inactivated by metabolization in the liver to about 50%. The rest is predominantly excreted into the gut (in part via the gallbladder, in part directly from blood vessels) and eliminated via the feces. About 10–15% are eliminated via the kidneys. The biological half-life is 14–22 (11–26[4]) hours in healthy people, up to 30 hours in those with kidney failure,[4] and significantly longer in those with liver disease.[5][52]
Chemistry
The drug is used in form of minocycline hydrochloride dihydrate,[52] which is sparingly soluble in water and slightly soluble in ethanol. Minocycline reacts acidic in aqueous solution.[5]
History
Minocycline was patented in 1961 and came into commercial use in 1971.[9] A topical foam for treatment of acne was approved in 2019.[3]
Society and culture
Brand names
- Minomycin
- Minostad (in Europe, for the treatment of acne)
- Akamin
- Minocin
- Minoderm
- Cyclimycin
- Arestin (1-mg doses administered locally into periodontal pockets, after scaling and root planing, for treatment of periodontal disease.)[53]
- Aknemin
- Solodyn (extended-release, for the treatment of acne)
- Dynacin
- Sebomin
- Mino-Tabs
- Acnamino
- Minopen (in Japan)
- Maracyn 2 (for treatment of bacterial infections in aquarium fish and amphibians)
- Quatrocin (in Syria)
- Minox (in Ireland)
- Minoz (in India and Romania)
- Divaine (in India)
- Vinocyclin 100 (100-mg dose approved for treatment of acne in Vietnam)
- Dentomycin (2% minocylcine gel for use in periodontal pockets)
- Amzeeq (4% foam, approved for treatment of acne United States)
- Zilxi (1.5% foam, approved for treatment of rosacea in the United States)
- Cleeravue-M
It is available as a generic medication.[7]
Research
Early research has found a tentative benefit from minocycline in schizophrenia,[54] with several trials underway.[55] A 2014 meta-analysis found minocycline may reduce negative and total symptom scores and was well tolerated.[56]
Research is examining the possible neuroprotective and anti-inflammatory effects of minocycline against the progression of a group of neurodegenerative disorders including multiple sclerosis, rheumatoid arthritis, Huntington's disease, and Parkinson's disease.[57][58][59][60] As mentioned above, minocycline harms ALS patients.
Minocycline is also known to indirectly inhibit inducible nitric oxide synthase.[61]
A trial found no difference between minocycline and placebo in people with Alzheimers' disease.[62] Minocycline also has been used as a "last-ditch" treatment for toxoplasmosis in AIDS patients.[63] Minocycline is somewhat neuroprotective in mouse models of Huntington's disease.[64]
A 2007 study reported the impact of the antibiotic minocycline on clinical and magnetic resonance imaging (MRI) outcomes and serum immune molecules in 40 MS patients over 24 months of open-label minocycline treatment. Despite a moderately high pretreatment relapse rate in the patient group prior to treatment (1.3/year pre-enrollment; 1.2/year during a three-month baseline period), no relapses occurred between months 6 and 24 on minocycline. Also, despite significant MRI disease-activity pretreatment (19/40 scans had gadolinium-enhancing activity during a three-month run-in), the only patient with gadolinium-enhancing lesions on MRI at 12 and 24 months was on half-dose minocycline. Levels of interleukin-12 (IL-12), which at high levels might antagonize the proinflammatory IL-12 receptor, were elevated over 18 months of treatment, as were levels of soluble vascular cell adhesion molecule-1 (VCAM-1). The activity of matrix metalloproteinase-9 was decreased by treatment. Clinical and MRI outcomes in this study were supported by systemic immunological changes and call for further investigation of minocycline in MS.[65][66][67]
Minocycline has been studied for treatment-resistant depression. According to a systematic review based on four clinical trials, "There is no significant difference with minocycline compared to placebo for depression not responding to first-line antidepressant therapy."[68]
In ongoing research and trial, minocycline demonstrated efficacy and seems a promising neuroprotective agent in acute stroke patients, especially in AIS subgroup. Further RCTs are needed to evaluate the efficacy and safety of minocycline among ICH patients.[69]
Several preclinical studies (in vitro cell cultures and animal models) suggest that minocycline may have otoprotective benefits. Animal models indicate it could potentially reduce noise-induced and blast-induced hearing loss, possibly by protecting hair cells and mitigating inflammation.[70][71] In vitro and animal studies also show minocycline may help decrease ototoxicity from certain drugs like gentamicin,[72] neomycin,[73] and cisplatin.[74][75]
Data from cellular and animal models
- PARP1 inhibition Ki = 13.8 nM[76]
- Neuroprotection IC50 = 10 nM[77]
- Microglia full inhibition = 20 nM[77]
- Suppression of the mouse's locomotor activity = 0.5 mg/kg[78]
References
- ↑ "Minocycline Use During Pregnancy". Drugs.com. 4 December 2018. Retrieved 16 May 2020.
- 1 2 "Minocin- minocycline hydrochloride injection". DailyMed. 28 July 2021. Retrieved 19 February 2023.
- 1 2 3 4 5 "Amzeeq- minocycline aerosol, foam". DailyMed. 25 January 2023. Retrieved 18 February 2023.
- 1 2 3 4 5 6 7 8 9 10 "Minocycline Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 23 March 2019.
- 1 2 3 4 5 6 7 8 9 10 Dinnendahl V, Fricke U, eds. (2010). "Minocyclin". Arzneistoff-Profile (in German). Vol. 7 (24 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-9846-3.
- ↑ "Minocycline". go.drugbank.com.
- 1 2 3 4 5 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 556. ISBN 9780857113382.
- ↑ "Minocycline Use During Pregnancy". Drugs.com. Retrieved 3 March 2019.
- 1 2 Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 489. ISBN 9783527607495.
- ↑ "First Generic Drug Approvals". U.S. Food and Drug Administration. 17 October 2022. Retrieved 28 November 2022.
- ↑ "The Top 300 of 2020". ClinCalc. Retrieved 7 October 2022.
- ↑ "Minocycline - Drug Usage Statistics". ClinCalc. Retrieved 7 October 2022.
- ↑ Strauss JS, Krowchuk DP, Leyden JJ, Lucky AW, Shalita AR, Siegfried EC, et al. (April 2007). "Guidelines of care for acne vulgaris management". Journal of the American Academy of Dermatology. 56 (4): 651–663. doi:10.1016/j.jaad.2006.08.048. PMID 17276540.
- ↑ "Minocycline, Doxycycline and Acne Vulgaris". ScienceOfAcne.com. Archived from the original on 7 August 2012. Retrieved 7 August 2012.
- ↑ Kircik LH (November 2010). "Doxycycline and minocycline for the management of acne: a review of efficacy and safety with emphasis on clinical implications". Journal of Drugs in Dermatology. 9 (11): 1407–1411. PMID 21061764.
- ↑ Hubbell CG, Hobbs ER, Rist T, White JW (December 1982). "Efficacy of minocycline compared with tetracycline in treatment of acne vulgaris". Archives of Dermatology. 118 (12): 989–992. doi:10.1001/archderm.1982.01650240033017. PMID 6216858.
- ↑ Eady EA, Gloor M, Leyden JJ (2003). "Propionibacterium acnes resistance: a worldwide problem". Dermatology. 206 (1): 54–56. doi:10.1159/000067822. PMID 12566805. S2CID 6111436.
- ↑ Ross JI, Snelling AM, Carnegie E, Coates P, Cunliffe WJ, Bettoli V, et al. (March 2003). "Antibiotic-resistant acne: lessons from Europe". The British Journal of Dermatology. 148 (3): 467–478. arXiv:0706.4406. doi:10.1046/j.1365-2133.2003.05067.x. hdl:10454/3069. PMID 12653738. S2CID 20838517.
- ↑ Rogers RL, Perkins J (September 2006). "Skin and soft tissue infections". Primary Care. 33 (3): 697–710. doi:10.1016/j.pop.2006.06.005. PMID 17088156.
- ↑ Fraser A, Gafter-Gvili A, Paul M, Leibovici L (March 2005). "Prophylactic use of antibiotics for prevention of meningococcal infections: systematic review and meta-analysis of randomised trials". European Journal of Clinical Microbiology & Infectious Diseases. 24 (3): 172–181. doi:10.1007/s10096-005-1297-7. PMID 15782277. S2CID 1259483.
- ↑ Bishburg E, Bishburg K (November 2009). "Minocycline--an old drug for a new century: emphasis on methicillin-resistant Staphylococcus aureus (MRSA) and Acinetobacter baumannii". International Journal of Antimicrobial Agents. 34 (5): 395–401. doi:10.1016/j.ijantimicag.2009.06.021. PMID 19665876.
- ↑ Copeland KF, Brooks JI (April 2010). "A novel use for an old drug: the potential for minocycline as anti-HIV adjuvant therapy". The Journal of Infectious Diseases. 201 (8): 1115–1117. doi:10.1086/651278. PMID 20205572.
- ↑ Mungroo MR, Khan NA, Siddiqui R (December 2020). "Mycobacterium leprae: Pathogenesis, diagnosis, and treatment options". Microbial Pathogenesis. 149: 104475. doi:10.1016/j.micpath.2020.104475. PMID 32931893. S2CID 221748544.
- ↑ U.S. National Library of Medicine (11 December 2009) 'Perioral dermatitis'. Retrieved 7 August 2010.
- ↑ Joks R, Durkin HG (December 2011). "Non-antibiotic properties of tetracyclines as anti-allergy and asthma drugs". Pharmacological Research. 64 (6): 602–609. doi:10.1016/j.phrs.2011.04.001. PMID 21501686.
- ↑ Greenwald RA (December 2011). "The road forward: the scientific basis for tetracycline treatment of arthritic disorders". Pharmacological Research. 64 (6): 610–613. doi:10.1016/j.phrs.2011.06.010. PMID 21723947.
- ↑ "Clinical Practice Guidelines: Rheumatoid Arthritis". American College of Rheumatology. Retrieved 13 May 2017.
- ↑ Sharma RK, Oliveira AC, Kim S, Rigatto K, Zubcevic J, Rathinasabapathy A, et al. (June 2018). "Involvement of Neuroinflammation in the Pathogenesis of Monocrotaline-Induced Pulmonary Hypertension". Hypertension. 71 (6): 1156–1163. doi:10.1161/HYPERTENSIONAHA.118.10934. PMC 5945302. PMID 29712738.
- ↑ Nonaka K, Nakazawa Y, Kotorii T (December 1983). "Effects of antibiotics, minocycline and ampicillin, on human sleep". Brain Research. 288 (1–2): 253–259. doi:10.1016/0006-8993(83)90101-4. PMID 6661620. S2CID 22726747.
- ↑ "MedlinePlus Drug Information: Minocycline Oral".
- ↑ Geria AN, Tajirian AL, Kihiczak G, Schwartz RA (2009). "Minocycline-induced skin pigmentation: an update". Acta Dermatovenerologica Croatica. 17 (2): 123–126. PMID 19595269.
- 1 2 "minocycline (Dynacin): Antibiotic Side Effects & Dosage". MedicineNet.
- ↑ Cohen PR (January 2004). "Medication-associated depersonalization symptoms: report of transient depersonalization symptoms induced by minocycline". Southern Medical Journal. 97 (1): 70–73. doi:10.1097/01.SMJ.0000083857.98870.98. PMID 14746427. S2CID 27125601.
- ↑ Mongey AB, Hess EV (March 2008). "Drug insight: autoimmune effects of medications-what's new?". Nature Clinical Practice. Rheumatology. 4 (3): 136–144. doi:10.1038/ncprheum0708. PMID 18200008. S2CID 205340777.
- 1 2 Ochsendorf F (2010). "Minocycline in acne vulgaris: benefits and risks". American Journal of Clinical Dermatology. 11 (5): 327–341. doi:10.2165/11319280-000000000-00000. PMID 20642295. S2CID 24501240.
- ↑ Sweet RL, Gibbs RS (2001). Infectious Diseases of the Female Genital Tract (4th ed.). Lippincott Williams & Wilkins. p. 635.
- ↑ Bauman, Neil (2010). Ototoxic Drugs Exposed (3 ed.). Integrity First Publications. pp. 435–436. ISBN 978-1-935939-00-9.
You can develop symptoms of ototoxicity after only one or two doses. These symptoms normally disappear a day or two after you stop taking this drug.
- ↑ "Minocycline Side Effects: Common, Severe, Long Term". Drugs.com. 27 December 2023. Archived from the original on 16 January 2024. Retrieved 16 January 2024.
Headache, dizziness, vertigo, and ataxia have been reported. These side effects were reversible within 3 to 48 hours of stopping therapy and occurred less often with low doses.
- ↑ Jacobson JA, Daniel B (October 1975). "Vestibular reactions associated with minocycline". Antimicrobial Agents and Chemotherapy. 8 (4): 453–6. doi:10.1128/AAC.8.4.453. PMC 429369. PMID 1081373.
- ↑ Friedman DI (2005). "Medication-induced intracranial hypertension in dermatology". American Journal of Clinical Dermatology. 6 (1): 29–37. doi:10.2165/00128071-200506010-00004. PMID 15675888. S2CID 28395784.
- ↑ Mechrgui M, Kanani S (August 2022). "The Ophthalmic Side Effects of Topiramate: A Review". Cureus. 14 (8): e28513. doi:10.7759/cureus.28513. PMC 9420653. PMID 36059357.
- ↑ Gough A, Chapman S, Wagstaff K, Emery P, Elias E (January 1996). "Minocycline induced autoimmune hepatitis and systemic lupus erythematosus-like syndrome". BMJ. 312 (7024): 169–172. doi:10.1136/bmj.312.7024.169. PMC 2349841. PMID 8563540.
- ↑ Krawitt EL (January 2006). "Autoimmune hepatitis". The New England Journal of Medicine. 354 (1): 54–66. doi:10.1056/NEJMra050408. PMID 16394302. S2CID 5361674.
- ↑ Friedman DI (2005). "Medication-induced intracranial hypertension in dermatology". American Journal of Clinical Dermatology. Springer Science and Business Media LLC. 6 (1): 29–37. doi:10.2165/00128071-200506010-00004. PMID 15675888. S2CID 28395784.
- ↑ Lefebvre N, Forestier E, Farhi D, Mahsa MZ, Remy V, Lesens O, et al. (May 2007). "Minocycline-induced hypersensitivity syndrome presenting with meningitis and brain edema: a case report". Journal of Medical Case Reports. 1: 22. doi:10.1186/1752-1947-1-22. PMC 1884162. PMID 17511865.
- 1 2 "Principles and methods for the assessment of nephrotoxicity associated with exposure to chemicals". Environmental health criteria: 119. World Health Organization (WHO). ISBN 92-4-157119-5. ISSN 0250-863X. 1991
- ↑ Piscitelli SC, Rodvold K (2005). Drug Interactions in Infectious Diseases. Humana Press. ISBN 978-1-58829-455-5.
- ↑ Drugs.com 'Minocycline Disease Interactions'. Retrieved 12 February 2017.
- ↑ Couzin J (November 2007). "Clinical research. ALS trial raises questions about promising drug". Science. 318 (5854): 1227. doi:10.1126/science.318.5854.1227a. PMID 18033854. S2CID 72187805.
- ↑ Thompson KG, Rainer BM, Antonescu C, Florea L, Mongodin EF, Kang S, Chien AL (February 2020). "Minocycline and Its Impact on Microbial Dysbiosis in the Skin and Gastrointestinal Tract of Acne Patients". Annals of Dermatology. 32 (1): 21–30. doi:10.5021/ad.2020.32.1.21. PMC 7992645. PMID 33911705.
- ↑ "Minocycline". mediQ. Retrieved 6 August 2020.
- 1 2 3 Haberfeld H, ed. (2020). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag. Minostad 50 mg-Kapseln.
- ↑ "How ARESTIN is supplied and dosed". OraPharma, Inc. Retrieved 1 January 2010.
- ↑ Dean OM, Data-Franco J, Giorlando F, Berk M (May 2012). "Minocycline: therapeutic potential in psychiatry". CNS Drugs. 26 (5): 391–401. doi:10.2165/11632000-000000000-00000. PMID 22486246.
- ↑ Arehart-Treichel J (17 August 2012). "Will Antibiotic Fulfill Its Psychosis-Fighting Promise?". Psychiatric News. 47 (16): 10. doi:10.1176/pn.47.16.psychnews_47_16_10-a.
- ↑ Oya K, Kishi T, Iwata N (September 2014). "Efficacy and tolerability of minocycline augmentation therapy in schizophrenia: a systematic review and meta-analysis of randomized controlled trials". Human Psychopharmacology. 29 (5): 483–491. doi:10.1002/hup.2426. PMID 25087702. S2CID 3564390.
- ↑ "Preliminary Study Shows Creatine and Minocycline May Warrant Further Study In Parkinson's Disease" (Press release). National Institute of Health. 23 February 2006.
- ↑ Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, et al. (July 2000). "Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease". Nature Medicine. 6 (7): 797–801. doi:10.1038/77528. PMID 10888929. S2CID 22681391.
- ↑ Tikka TM, Koistinaho JE (June 2001). "Minocycline provides neuroprotection against N-methyl-D-aspartate neurotoxicity by inhibiting microglia". Journal of Immunology. 166 (12): 7527–7533. doi:10.4049/jimmunol.166.12.7527. PMID 11390507.
- ↑ Nirmalananthan N, Greensmith L (December 2005). "Amyotrophic lateral sclerosis: recent advances and future therapies". Current Opinion in Neurology. 18 (6): 712–719. doi:10.1097/01.wco.0000187248.21103.c5. PMID 16280684. S2CID 3255995.
- ↑ Amin AR, Attur MG, Thakker GD, Patel PD, Vyas PR, Patel RN, et al. (November 1996). "A novel mechanism of action of tetracyclines: effects on nitric oxide synthases". Proceedings of the National Academy of Sciences of the United States of America. 93 (24): 14014–14019. Bibcode:1996PNAS...9314014A. doi:10.1073/pnas.93.24.14014. PMC 19486. PMID 8943052.
- ↑ Howard R, Zubko O, Bradley R, Harper E, Pank L, O'Brien J, et al. (February 2020). "Minocycline at 2 Different Dosages vs Placebo for Patients With Mild Alzheimer Disease: A Randomized Clinical Trial". JAMA Neurology. 77 (2): 164–174. doi:10.1001/jamaneurol.2019.3762. PMC 6865324. PMID 31738372.
- ↑ Lacassin F, Schaffo D, Perronne C, Longuet P, Leport C, Vilde JL (January 1995). "Clarithromycin-minocycline combination as salvage therapy for toxoplasmosis in patients infected with human immunodeficiency virus". Antimicrobial Agents and Chemotherapy. 39 (1): 276–277. doi:10.1128/AAC.39.1.276. PMC 162527. PMID 7695324.
- ↑ Beal MF, Ferrante RJ (May 2004). "Experimental therapeutics in transgenic mouse models of Huntington's disease". Nature Reviews. Neuroscience. 5 (5): 373–384. doi:10.1038/nrn1386. PMID 15100720. S2CID 19496441.
- ↑ Zemke D, Majid A (2004). "The potential of minocycline for neuroprotection in human neurologic disease". Clinical Neuropharmacology. 27 (6): 293–298. doi:10.1097/01.wnf.0000150867.98887.3e. PMID 15613934. S2CID 30431947.
- ↑ Maier K, Merkler D, Gerber J, Taheri N, Kuhnert AV, Williams SK, et al. (March 2007). "Multiple neuroprotective mechanisms of minocycline in autoimmune CNS inflammation". Neurobiology of Disease. 25 (3): 514–525. doi:10.1016/j.nbd.2006.10.022. PMID 17239606. S2CID 39628457.
- ↑ Popovic N, Schubart A, Goetz BD, Zhang SC, Linington C, Duncan ID (February 2002). "Inhibition of autoimmune encephalomyelitis by a tetracycline". Annals of Neurology. 51 (2): 215–223. doi:10.1002/ana.10092. PMID 11835378. S2CID 21209994.
- ↑ Shamim MA, Manna S, Dwivedi P, Swami MK, Sahoo S, Shukla R, et al. (November 2023). "Minocycline in depression not responding to first-line therapy: A systematic review and meta-analysis". Medicine. 102 (45): e35937. doi:10.1097/MD.0000000000035937. PMC 10637431. PMID 37960804.
- ↑ Malhotra K, Chang JJ, Khunger A, Blacker D, Switzer JA, Goyal N, et al. (August 2018). "Minocycline for acute stroke treatment: a systematic review and meta-analysis of randomized clinical trials". Journal of Neurology. 265 (8): 1871–1879. doi:10.1007/s00415-018-8935-3. hdl:10757/624615. PMID 29948247. S2CID 49431206.
- ↑ Zhang J, Song YL, Tian KY, Qiu JH (February 2017). "Minocycline attenuates noise-induced hearing loss in rats". Neuroscience Letters. 639: 31–35. doi:10.1016/j.neulet.2016.12.039. PMID 28007648. S2CID 38379616.
- ↑ Perumal V, Ravula AR, Shao N, Chandra N (January 2023). "Effect of minocycline and its nano-formulation on central auditory system in blast-induced hearing loss rat model". Journal of Otology. 18 (1): 38–48. doi:10.1016/j.joto.2022.09.002. PMC 9937842. PMID 36820161.
- ↑ Wei X, Zhao L, Liu J, Dodel RC, Farlow MR, Du Y (2005). "Minocycline prevents gentamicin-induced ototoxicity by inhibiting p38 MAP kinase phosphorylation and caspase 3 activation". Neuroscience. 131 (2): 513–21. doi:10.1016/j.neuroscience.2004.11.014. PMID 15708492. S2CID 3125930.
- ↑ Robinson AM, Vujanovic I, Richter CP (2015). "Minocycline protection of neomycin induced hearing loss in gerbils". BioMed Research International. 2015: 934158. doi:10.1155/2015/934158. PMC 4407513. PMID 25950003.
- ↑ Lee CK, Shin JI, Cho YS (June 2011). "Protective Effect of Minocycline Against Cisplatin-induced Ototoxicity". Clinical and Experimental Otorhinolaryngology. 4 (2): 77–82. doi:10.3342/ceo.2011.4.2.77. PMC 3109331. PMID 21716954.
- ↑ Du B, Zhang Y, Tang Y, Wang P (May 2011). "Minocycline attenuates ototoxicity and enhances antitumor activity of cisplatin treatment in vitro". Otolaryngology–Head and Neck Surgery. 144 (5): 719–25. doi:10.1177/0194599810395090. PMID 21493367. S2CID 25994657.
- ↑ Alano CC, Kauppinen TM, Valls AV, Swanson RA (June 2006). "Minocycline inhibits poly(ADP-ribose) polymerase-1 at nanomolar concentrations". Proceedings of the National Academy of Sciences of the United States of America. 103 (25): 9685–9690. Bibcode:2006PNAS..103.9685A. doi:10.1073/pnas.0600554103. PMC 1480467. PMID 16769901.
- 1 2 Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J (April 2001). "Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia". The Journal of Neuroscience. 21 (8): 2580–2588. doi:10.1523/JNEUROSCI.21-08-02580.2001. PMC 6762519. PMID 11306611.
- ↑ "minomycin-if" (PDF). Archived from the original (PDF) on 8 September 2017. Retrieved 8 September 2017.