 | |
 | |
| Clinical data | |
|---|---|
| Other names | Biacalein; Noroxylin |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.164.911 |
| Chemical and physical data | |
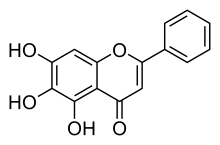
| Formula | C15H10O5 |
| Molar mass | 270.240 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Baicalein (5,6,7-trihydroxyflavone) is a flavone, a type of flavonoid, originally isolated from the roots of Scutellaria baicalensis and Scutellaria lateriflora. It is also reported in Oroxylum indicum (Indian trumpetflower) and Thyme.[1] It is the aglycone of baicalin. Baicalein is one of the active ingredients of Sho-Saiko-To, which is a Chinese classic herbal formula, and listed in Japan as Kampo medicine.
Baicalein, along with its analogue baicalin, is a positive allosteric modulator of the benzodiazepine site and/or a non-benzodiazepine site of the GABAA receptor but with an affinity over 250× lower than diazepam.[2][3][4][5][6][7] It displays subtype selectivity for α2 and α3 subunit-containing GABAA receptors.[8] In accordance, baicalein shows anxiolytic effects in mice without incidence of sedation or myorelaxation.[7][8][9] It is thought that baicalein, along with other flavonoids, may underlie the anxiolytic effects of S. baicalensis and S. lateriflora.[10][11] Baicalein is also an antagonist of the estrogen receptor, or an antiestrogen.[11]
The flavonoid has been shown to inhibit certain types of lipoxygenases[12] and act as an anti-inflammatory agent.[13] It has antiproliferative effects on ET-1-induced proliferation of pulmonary artery smooth muscle cell proliferation via inhibition of TRPC1 channel expression.[14] Possible antidepressant effects have also been attributed to baicalein in animal research.[15]
Baicalein is an inhibitor of CYP2C9,[16] an enzyme of the cytochrome P450 system that metabolizes drugs in the body.
A derivative of baicalin is a known prolyl endopeptidase inhibitor.[17]
Baicalein has been shown to inhibit Staphylococcus aureus biofilm formation and the quorum sensing system in vitro.[18]
It has also been shown to be effective in vitro against all forms of Borrelia burgdorferi and Borrelia garinii.[19]
Glycosides
See also
References
- ↑ Matsumoto T (2008). Phytochemistry Research Progress. Nova Publishers. ISBN 9781604562323.
- ↑ Wang H, Hui KM, Xu S, Chen Y, Wong JT, Xue H (December 2002). "Two flavones from Scutellaria baicalensis Georgi and their binding affinities to the benzodiazepine site of the GABAA receptor complex". Die Pharmazie. 57 (12): 857–858. PMID 12561253.
- ↑ Hui KM, Wang XH, Xue H (February 2000). "Interaction of flavones from the roots of Scutellaria baicalensis with the benzodiazepine site". Planta Medica. 66 (1): 91–93. doi:10.1055/s-0029-1243121. PMID 10705749. S2CID 260249283.
- ↑ Zhang SQ, Obregon D, Ehrhart J, Deng J, Tian J, Hou H, et al. (September 2013). "Baicalein reduces β-amyloid and promotes nonamyloidogenic amyloid precursor protein processing in an Alzheimer's disease transgenic mouse model". Journal of Neuroscience Research. 91 (9): 1239–1246. doi:10.1002/jnr.23244. PMC 3810722. PMID 23686791.
- ↑ Liao JF, Wang HH, Chen MC, Chen CC, Chen CF (August 1998). "Benzodiazepine binding site-interactive flavones from Scutellaria baicalensis root". Planta Medica. 64 (6): 571–572. doi:10.1055/s-2006-957517. PMID 9776664. S2CID 260251315.
- ↑ Roberts AA (2004). "Testing efficacy of natural anxiolytic compounds". In Cooper EL, Yamaguchi N (eds.). Complementary and Alternative Approaches to Biomedicine. Advances in Experimental Medicine and Biology. Vol. 546. pp. 181–191. doi:10.1007/978-1-4757-4820-8_13. ISBN 978-1-4419-3441-3. PMID 15584374.
- 1 2 de Carvalho RS, Duarte FS, de Lima TC (August 2011). "Involvement of GABAergic non-benzodiazepine sites in the anxiolytic-like and sedative effects of the flavonoid baicalein in mice". Behavioural Brain Research. 221 (1): 75–82. doi:10.1016/j.bbr.2011.02.038. PMID 21377498. S2CID 45903577.
- 1 2 Wang F, Xu Z, Ren L, Tsang SY, Xue H (December 2008). "GABA A receptor subtype selectivity underlying selective anxiolytic effect of baicalin". Neuropharmacology. 55 (7): 1231–1237. doi:10.1016/j.neuropharm.2008.07.040. PMID 18723037. S2CID 20133964.
- ↑ Liao JF, Hung WY, Chen CF (March 2003). "Anxiolytic-like effects of baicalein and baicalin in the Vogel conflict test in mice". European Journal of Pharmacology. 464 (2–3): 141–146. doi:10.1016/s0014-2999(03)01422-5. PMID 12620506.
- ↑ Awad R, Arnason JT, Trudeau V, Bergeron C, Budzinski JW, Foster BC, Merali Z (November 2003). "Phytochemical and biological analysis of skullcap (Scutellaria lateriflora L.): a medicinal plant with anxiolytic properties". Phytomedicine. 10 (8): 640–649. doi:10.1078/0944-7113-00374. PMID 14692724.
- 1 2 Schwartz S (9 January 2008). "Native American Psychoactive Herbs". Psychoactive Herbs in Veterinary Behavior Medicine. John Wiley & Sons. pp. 139–. ISBN 978-0-470-34434-7.
- ↑ Deschamps JD, Kenyon VA, Holman TR (June 2006). "Baicalein is a potent in vitro inhibitor against both reticulocyte 15-human and platelet 12-human lipoxygenases". Bioorganic & Medicinal Chemistry. 14 (12): 4295–4301. doi:10.1016/j.bmc.2006.01.057. PMID 16500106. S2CID 645610.
- ↑ Hsieh CJ, Hall K, Ha T, Li C, Krishnaswamy G, Chi DS (November 2007). "Baicalein inhibits IL-1beta- and TNF-alpha-induced inflammatory cytokine production from human mast cells via regulation of the NF-kappaB pathway". Clinical and Molecular Allergy. 5 (1): 5. doi:10.1186/1476-7961-5-5. PMC 2206049. PMID 18039391.
- ↑ Lin YL, Lin RJ, Shen KP, Dai ZK, Chen IJ, Wu JR, Wu BN (November 2011). "Baicalein, isolated from Scutellaria baicalensis, protects against endothelin-1-induced pulmonary artery smooth muscle cell proliferation via inhibition of TRPC1 channel expression". Journal of Ethnopharmacology. 138 (2): 373–381. doi:10.1016/j.jep.2011.09.014. PMID 21963569.
- ↑ Xiong Z, Jiang B, Wu PF, Tian J, Shi LL, Gu J, et al. (2011). "Antidepressant effects of a plant-derived flavonoid baicalein involving extracellular signal-regulated kinases cascade". Biological & Pharmaceutical Bulletin. 34 (2): 253–259. doi:10.1248/bpb.34.253. PMID 21415537.
- ↑ Si D, Wang Y, Zhou YH, Guo Y, Wang J, Zhou H, et al. (March 2009). "Mechanism of CYP2C9 inhibition by flavones and flavonols" (PDF). Drug Metabolism and Disposition. 37 (3): 629–634. doi:10.1124/dmd.108.023416. PMID 19074529. S2CID 285706. Archived from the original (PDF) on 2008-12-17. Retrieved 2009-02-19.
- ↑ Tarragó T, Kichik N, Claasen B, Prades R, Teixidó M, Giralt E (August 2008). "Baicalin, a prodrug able to reach the CNS, is a prolyl oligopeptidase inhibitor". Bioorganic & Medicinal Chemistry. 16 (15): 7516–7524. doi:10.1016/j.bmc.2008.04.067. PMID 18650094.
- ↑ Chen Y, Liu T, Wang K, Hou C, Cai S, Huang Y, et al. (April 2016). "Baicalein Inhibits Staphylococcus aureus Biofilm Formation and the Quorum Sensing System In Vitro". PLOS ONE. 11 (4): e0153468. Bibcode:2016PLoSO..1153468C. doi:10.1371/journal.pone.0153468. PMC 4851419. PMID 27128436.
- ↑ Goc A, Niedzwiecki A, Rath M (December 2015). "In vitro evaluation of antibacterial activity of phytochemicals and micronutrients against Borrelia burgdorferi and Borrelia garinii". Journal of Applied Microbiology. 119 (6): 1561–1572. doi:10.1111/jam.12970. PMC 4738477. PMID 26457476.