 | |
| Names | |
|---|---|
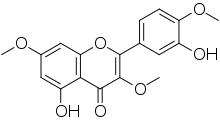
| IUPAC name
3′,5-Dihydroxy-3,4′,7-trimethoxyflavone | |
| Systematic IUPAC name
5-Hydroxy-2-(3-hydroxy-4-methoxyphenyl)-3,7-dimethoxy-4H-1-benzopyran-4-one | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H16O7 | |
| Molar mass | 344.319 g·mol−1 |
| Density | 1.454 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Ayanin is an O-methylated flavonol, a type of flavonoid. It is the 3,7,4'-tri-O-methylated derivative of quercetin.
It can be found in Croton schiedeanus. It can also be synthesized.[1]
Biosynthesis
The enzyme 3,7-dimethylquercetin 4'-O-methyltransferase uses S-adenosyl methionine and rhamnazin to produce S-adenosylhomocysteine and ayanin.
References
- ↑ Rao, Koppaka V.; Owoyale, Jacob A. (1976). "Partial methylation of quercetin: Direct synthesis of tamarixetin, ombuin and ayanin". Journal of Heterocyclic Chemistry. 13 (6): 1293–1295. doi:10.1002/jhet.5570130629.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.