 | |
| Names | |
|---|---|
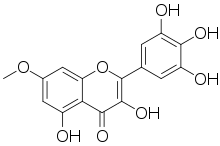
| IUPAC name
3,5,3′,4′,5′-Pentahydroxy-7-methoxyflavone | |
| Systematic IUPAC name
3,5-Dihydroxy-7-methoxy-2-(3,4,5-trihydroxyphenyl)-4H-1-benzopyran-4-one | |
| Other names
7-Methylmyricetin | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H12O8 | |
| Molar mass | 332.264 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Europetin is an O-methylated flavonol. It can be found in Plumbago europaea[1] and it can be prepared synthetically.[2]
References
- ↑ Europetin on metabolomics.jp
- ↑ Sarma, P.N., Srimannarayana, G. & Subba Rao, N.V. (1974). "Synthesis of naturally occurring partial methyl ethers of myricetin". Proc. Indian Acad. Sci. 80 (4): 168–173. doi:10.1007/BF03046674. S2CID 92325935.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.