 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
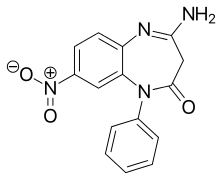
| Formula | C15H12N4O3 |
| Molar mass | 296.286 g·mol−1 |
| |
| (verify) | |
CP-1414S is an experimental drug first made by a team in Germany.[1] It is a benzodiazepine derivative. CP-1414S is a 1,5-benzodiazepine, with the nitrogen atoms located at positions 1 and 5 of the diazepine ring, and so is most closely related to other 1,5-benzodiazepines such as clobazam.
CP-1414S has primarily anxiolytic and anticonvulsant effects.[2] Its potency is roughly equal to that of clobazam, but with more pronounced sedation.[3]
See also
References
- ↑ US Patent 3766169 PROCESS FOR THE PREPARATION OF 3-AMINOMETHYLIDENE-1,5-BENZODIAZEPINE-2,4-(3H,5H)-DIONES
- ↑ Carli M, Ballabio M, Caccia S, Garattini S, Samanin R (1981). "Studies on some pharmacological activities of 7-nitro-2-amino-5-phenyl-3H-1,5-benzodiazepine (CP 1414 S) in the rat. A comparison with diazepam". Arzneimittel-Forschung. 31 (10): 1721–3. PMID 6119091.
- ↑ Mennini T, Garattini S (November 1982). "Benzodiazepine receptors': correlation with pharmacological responses in living animals". Life Sciences. 31 (19): 2025–35. doi:10.1016/0024-3205(82)90094-7. PMID 6129557.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.