 | |
 | |
| Names | |
|---|---|
| IUPAC name
Guanosine 5′-(tetrahydrogen triphosphate) | |
| Systematic IUPAC name
O1-{[(2R,3S,4R,5R)-5-(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl} tetrahydrogen triphosphate | |
| Other names
guanosine triphosphate, 9-β-D-ribofuranosylguanine-5'-triphosphate, 9-β-D-ribofuranosyl-2-amino-6-oxo-purine-5'-triphosphate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.498 |
| KEGG | |
| MeSH | Guanosine+triphosphate |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H16N5O14P3 | |
| Molar mass | 523.180 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
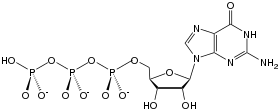
Guanosine-5'-triphosphate (GTP) is a purine nucleoside triphosphate. It is one of the building blocks needed for the synthesis of RNA during the transcription process. Its structure is similar to that of the guanosine nucleoside, the only difference being that nucleotides like GTP have phosphates on their ribose sugar. GTP has the guanine nucleobase attached to the 1' carbon of the ribose and it has the triphosphate moiety attached to ribose's 5' carbon.
It also has the role of a source of energy or an activator of substrates in metabolic reactions, like that of ATP, but more specific. It is used as a source of energy for protein synthesis and gluconeogenesis.
GTP is essential to signal transduction, in particular with G-proteins, in second-messenger mechanisms where it is converted to guanosine diphosphate (GDP) through the action of GTPases.
Uses
Energy transfer
GTP is involved in energy transfer within the cell. For instance, a GTP molecule is generated by one of the enzymes in the citric acid cycle. This is tantamount to the generation of one molecule of ATP, since GTP is readily converted to ATP with nucleoside-diphosphate kinase (NDK).[1]
Genetic translation
During the elongation stage of translation, GTP is used as an energy source for the binding of a new amino-bound tRNA to the A site of the ribosome. GTP is also used as an energy source for the translocation of the ribosome towards the 3' end of the mRNA.[2]
Microtubule dynamic instability
During microtubule polymerization, each heterodimer formed by an alpha and a beta tubulin molecule carries two GTP molecules, and the GTP is hydrolyzed to GDP when the tubulin dimers are added to the plus end of the growing microtubule. Such GTP hydrolysis is not mandatory for microtubule formation, but it appears that only GDP-bound tubulin molecules are able to depolymerize. Thus, a GTP-bound tubulin serves as a cap at the tip of microtubule to protect from depolymerization; and, once the GTP is hydrolyzed, the microtubule begins to depolymerize and shrink rapidly.[3]
Mitochondrial function
The translocation of proteins into the mitochondrial matrix involves the interactions of both GTP and ATP. The importing of these proteins plays an important role in several pathways regulated within the mitochondria organelle,[4] such as converting oxaloacetate to phosphoenolpyruvate (PEP) in gluconeogenesis.
Precursor for synthesis of riboflavin
GTP, in combination with ribulose 5-phosphate, are the precursor compounds for the synthesis of riboflavin (vitamin B2).[5]
Biosynthesis
In the cell, GTP is synthesised through many processes including:
- as a byproduct of the Succinyl-CoA to Succinate conversion catalysed by the Succinyl-CoA synthetase enzyme as part of the Krebs cycle;[1]
- through exchanges of phosphate groups from ATP molecules by the Nucleoside-diphosphate kinase, an enzyme tasked with maintaining an equilibrium between the concentrations of different nucleoside triphosphates.[1]
See also
References
- 1 2 3 Berg, JM; JL Tymoczko; L Stryer (2002). Biochemistry (5th ed.). WH Freeman and Company. pp. 476. ISBN 0-7167-4684-0.
- ↑ Solomon, EP; LR Berg; DW Martin (2005). Biology (7th ed.). pp. 244–245.
- ↑ Gwen V. Childs. "Microtubule structure". cytochemistry.net. Archived from the original on 2010-02-15.
- ↑ Sepuri, Naresh Babu V.; Norbert Schülke; Debkumar Pain (16 January 1998). "GTP Hydrolysis Is Essential for Protein Import into the Mitochondrial Matrix". Journal of Biological Chemistry. 273 (3): 1420–1424. doi:10.1074/jbc.273.3.1420. PMID 9430677.
- ↑ Merrill AH, McCormick DB (2020). "Riboflavin". In BP Marriott, DF Birt, VA Stallings, AA Yates (eds.). Present Knowledge in Nutrition, Eleventh Edition. London, United Kingdom: Academic Press (Elsevier). pp. 189–208. ISBN 978-0-323-66162-1.
External links
- GTP bound to proteins in the PDB