 | |
| Clinical data | |
|---|---|
| Trade names | Ikorel, others |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 75 to 80% |
| Protein binding | 25% |
| Metabolism | Liver |
| Elimination half-life | 1 hour |
| Excretion | Kidney (21%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.059.541 |
| Chemical and physical data | |
| Formula | C8H9N3O4 |
| Molar mass | 211.177 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Nicorandil is a vasodilator drug used to treat angina.
Angina is chest pain that results from episodes of transient myocardial ischemia. This can be caused by diseases such as atherosclerosis, coronary artery disease and aortic stenosis. Angina commonly arises from vasospasm of the coronary arteries. There are multiple mechanisms causing the increased smooth muscle contraction involved in coronary vasospasm, including increased Rho-kinase activity. Increased levels of Rho-kinase inhibit myosin phosphatase activity, leading to increased calcium sensitivity and hypercontraction.[1] Rho-kinase also decreases nitric oxide synthase activity, which reduces nitric oxide concentrations.[2] Lower levels of nitric oxide are present in spastic coronary arteries.[3] L-type calcium channel expression increases in spastic vascular smooth muscle cells, which could result in excessive calcium influx, and hypercontraction.[4]
It was patented in 1976 and approved for medical use in 1983.[5]
Side effects
Side effects listed in the British National Formulary include flushing, palpitations, weakness and vomiting. More recently, perianal, ileal and peristomal ulceration has been reported as a side effect. Anal ulceration is now included in the British National Formulary as a reported side effect. Other side effects include severe migraine, toothache, and nasal congestion.
Mechanism of action
Nicorandil is an anti-angina medication that has the dual properties of a nitrate and ATP-sensitive K+
channel opener.[6] In humans, the nitrate action of nicorandil dilates the large coronary arteries at low plasma concentrations.[6] At high plasma concentrations nicorandil reduces coronary vascular resistance, which is associated with increased ATP-sensitive K+ channel (KATP) opening.[6]
Nicorandil stimulates guanylate cyclase to increase formation of cyclic GMP (cGMP).[7] cGMP activates protein kinase G (PKG), which phosphorylates and inhibits GTPase RhoA and decreases Rho-kinase activity.[7] Reduced Rho-kinase activity permits an increase in myosin phosphatase activity, decreasing the calcium sensitivity of the smooth muscle.[7]
PKG also activates the sarcolemma calcium pump to remove activating calcium.[8] PKG acts on K+
channels to promote K+ efflux and the ensuing hyperpolarization inhibits voltage-gated calcium channels.[6] Overall, this leads to relaxation of the smooth muscle and coronary vasodilation.
The effect of nicorandil as a vasodilator is mainly attributed to its nitrate property.[6] Yet, nicorandil is effective in cases where nitrates, such as nitroglycerine, are not effective.[6] Studies show that this is due to its KATP channel agonist action which causes pharmacological preconditioning and provides cardioprotective effects against ischemia.[6] Nicorandil activates KATP channels in the mitochondria of the myocardium, which appears to relay the cardioprotective effects, although the mechanism is still unclear.[9] In experimental animal models of the Long QT syndrome, Nicorandil normalizes the prolonged cardiac action potential duration and the QT interval.[10]
Society and culture
Brand names
Nicorandil is marketed under the brand names Ikorel (in the United Kingdom, Australia and most of Europe), Angedil (in Romania, Poland), Dancor (in Switzerland), Nikoran, PCA (in India), Aprior (in the Philippines), Nitorubin (in Japan), and Sigmart (in Japan, South Korea, Taiwan and China). Nicorandil is not available in the United States.
Synthesis
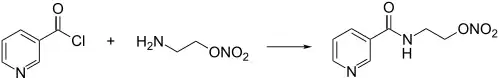
Amide reaction between Nicotinoyl Chloride [10400-19-8] & 2-Aminoethyl Nitrate [646-02-6].
The reaction of N-(2-Hydroxyethyl)Nicotinamide [6265-73-2] with nitric acid gives nicorandil.
See also
References
- ↑ Kandabashi T, Shimokawa H, Miyata K, Kunihiro I, Kawano Y, Fukata Y, et al. (March 2000). "Inhibition of myosin phosphatase by upregulated rho-kinase plays a key role for coronary artery spasm in a porcine model with interleukin-1beta". Circulation. 101 (11): 1319–1323. doi:10.1161/01.cir.101.11.1319. PMID 10725293.
- ↑ Takemoto M, Sun J, Hiroki J, Shimokawa H, Liao JK (July 2002). "Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase". Circulation. 106 (1): 57–62. doi:10.1161/01.cir.0000020682.73694.ab. PMID 12093770.
- ↑ Kugiyama K, Yasue H, Okumura K, Ogawa H, Fujimoto K, Nakao K, et al. (August 1996). "Nitric oxide activity is deficient in spasm arteries of patients with coronary spastic angina". Circulation. 94 (3): 266–271. doi:10.1161/01.cir.94.3.266. PMID 8759065.
- ↑ Kuga T, Shimokawa H, Hirakawa Y, Kadokami Y, Arai Y, Fukumoto Y, et al. (May 2000). "Increased expression of L-type calcium channels in vascular smooth muscle cells at spastic site in a porcine model of coronary artery spasm". Journal of Cardiovascular Pharmacology. 35 (5): 822–828. doi:10.1097/00005344-200005000-00021. PMID 10813387.
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 454. ISBN 9783527607495.
- 1 2 3 4 5 6 7 Nakae I, Matsumoto T, Horie H, Yokohama H, Omura T, Minai K, et al. (June 2000). "Effects of intravenous nicorandil on coronary circulation in humans: plasma concentration and action mechanism". Journal of Cardiovascular Pharmacology. 35 (6): 919–925. doi:10.1097/00005344-200006000-00014. PMID 10836727.
- 1 2 3 Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Smolenski A, Lohmann SM, Bertoglio J, et al. (July 2000). "Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle". The Journal of Biological Chemistry. 275 (28): 21722–21729. doi:10.1074/jbc.M000753200. PMID 10783386.
- ↑ Vrolix M, Raeymaekers L, Wuytack F, Hofmann F, Casteels R (November 1988). "Cyclic GMP-dependent protein kinase stimulates the plasmalemmal Ca2+ pump of smooth muscle via phosphorylation of phosphatidylinositol". The Biochemical Journal. 255 (3): 855–863. doi:10.1042/bj2550855. PMC 1135320. PMID 2850801.
- ↑ Liu Y, Sato T, O'Rourke B, Marban E (June 1998). "Mitochondrial ATP-dependent potassium channels: novel effectors of cardioprotection?". Circulation. 97 (24): 2463–2469. doi:10.1161/01.cir.97.24.2463. PMID 9641699.
- ↑ Biermann J, Wu K, Odening KE, Asbach S, Koren G, Peng X, et al. (January 2011). "Nicorandil normalizes prolonged repolarisation in the first transgenic rabbit model with Long-QT syndrome 1 both in vitro and in vivo". European Journal of Pharmacology. 650 (1): 309–316. doi:10.1016/j.ejphar.2010.10.016. PMC 2997896. PMID 20959120.
- ↑ DE 2714713, issued 16 September 1993, assigned to Chugai Seiyaku Kabushiki Kaisha
- ↑ US 4200640, published 1980, assigned to Chugai Seiyaku Kabushiki Kaisha
- ↑ WO 2010000673, published 2010, assigned to Politechnika Lódzka, Uniwersytet Jagiellonski
- ↑ WO 2012089769, issued 2012, assigned to Procos S.P.A.
- ↑ CN 111269175, issued 2020, assigned to Zhangjiagang Jiuli New Material Technology Co Ltd.
- ↑ CN 110845403, issued 2020, assigned to Beijing Voban Pharmaceutical Co Ltd.
Further reading
- Kukovetz WR, Holzmann S, Pöch G (1992). "Molecular mechanism of action of nicorandil". Journal of Cardiovascular Pharmacology. 20 Suppl 3 (Suppl 3): S1–S7. doi:10.1097/00005344-199206203-00002. PMID 1282168. S2CID 39747040.
- Tripathi KD (2004). "Chapter 37". Essentials of Medical Pharmacology. p. 499. ISBN 8180611876.
.svg.png.webp)