 | |
| Clinical data | |
|---|---|
| Trade names | Betafrine |
| Other names | Isopropylnorsynephrine, Isopropyloctopamine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C11H17NO2 |
| Molar mass | 195.262 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
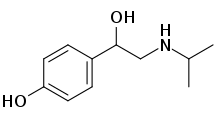
Deterenol (also known as Isopropylnorsynephrine and Isopropyloctopamine; trade name Betaphrine) is a stimulant drug which acts as a beta agonist. It has been found as an ingredient of dietary supplement products, but is banned in most countries due to risk of cardiac arrest.[1][2][3][4][5]
See also
References
- ↑ Anderson WG (June 1983). "The sympathomimetic activity of N-isopropyloctopamine in vitro". The Journal of Pharmacology and Experimental Therapeutics. 225 (3): 553–8. PMID 6306210.
- ↑ Mercader J, Wanecq E, Chen J, Carpéné C (September 2011). "Isopropylnorsynephrine is a stronger lipolytic agent in human adipocytes than synephrine and other amines present in Citrus aurantium". Journal of Physiology and Biochemistry. 67 (3): 443–52. doi:10.1007/s13105-011-0078-2. PMID 21336650. S2CID 22449550.
- ↑ Venhuis B, Keizers P, van Riel A, de Kaste D (June 2014). "A cocktail of synthetic stimulants found in a dietary supplement associated with serious adverse events". Drug Testing and Analysis. 6 (6): 578–81. doi:10.1002/dta.1664. PMID 24802503.
- ↑ Zhao J, Wang M, Avula B, Khan IA (March 2018). "Detection and quantification of phenethylamines in sports dietary supplements by NMR approach". Journal of Pharmaceutical and Biomedical Analysis. 151: 347–355. doi:10.1016/j.jpba.2018.01.025. PMID 29413984. S2CID 46837518.
- ↑ Cohen PA, Travis JC, Vanhee C, Ohana D, Venhuis BJ (March 2021). "Nine prohibited stimulants found in sports and weight loss supplements: deterenol, phenpromethamine (Vonedrine), oxilofrine, octodrine, beta-methylphenylethylamine (BMPEA), 1,3-dimethylamylamine (1,3-DMAA), 1,4-dimethylamylamine (1,4-DMAA), 1,3-dimethylbutylamine (1,3-DMBA) and higenamine". Clinical Toxicology. 59 (11): 975–981. doi:10.1080/15563650.2021.1894333. PMID 33755516.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.