 | |
| Names | |
|---|---|
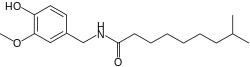
| Preferred IUPAC name
N-[(4-Hydroxy-3-methoxyphenyl)methyl]-8-methylnonanamide | |
| Other names
Dihydrocapsaicin, 6,7-Dihydrocapsaicin, 8-Methyl-N-vanillylnonanamide, Vanillylamide of 8-methylnonanoic acid, DHC, CCRIS 1589 | |
| Identifiers | |
3D model (JSmol) |
|
| 2815150 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.115.366 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H29NO3 | |
| Molar mass | 307.43 g/mol |
| Appearance | White to off-white solid |
| Sparingly soluble | |
| Hazards | |
| GHS labelling: | |
 | |
| Danger | |
| H301, H315, H319, H335 | |
| P261, P264, P270, P271, P280, P301+P310, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
| Dihydrocapsaicin | |
|---|---|
| Heat | Above peak (pure Dihydrocapsaicin is toxic) |
| Scoville scale | 16,000,000[1] SHU |

MS/MS spectra of standard dihydrocapsaicin (A) and from sample extract (B). Sample B confirms the compound was found in prehispanic pottery from Mexico. See here for details doi:10.1371/journal.pone.0079013.g005
Dihydrocapsaicin is a capsaicinoid and analog and congener of capsaicin in chili peppers (Capsicum). Like capsaicin, it is an irritant. It accounts for about 22% of the total capsaicinoid mixture[2] and has the same pungency as capsaicin.[1] Pure dihydrocapsaicin is a lipophilic colorless odorless crystalline to waxy compound. It is soluble in dimethyl sulfoxide and 100% ethanol.
See also
References
- 1 2 Govindarajan, Sathyanarayana (1991). "Capsicum — Production, Technology, Chemistry, and Quality. Part V. Impact on Physiology, Pharmacology, Nutrition, and Metabolism; Structure, Pungency, Pain, and Desensitization Sequences". Critical Reviews in Food Science and Nutrition. 29 (6): 435–474. doi:10.1080/10408399109527536. PMID 2039598.
- ↑ Bennett DJ, Kirby GW (1968). "Constitution and biosynthesis of capsaicin". J. Chem. Soc. C: 442. doi:10.1039/j39680000442.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
