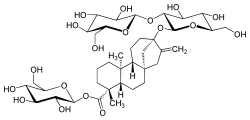
Steviol glycosides are the chemical compounds responsible for the sweet taste of the leaves of the South American plant Stevia rebaudiana (Asteraceae) and the main ingredients (or precursors) of many sweeteners marketed under the generic name stevia and several trade names. They also occur in the related species S. phlebophylla (but in no other species of Stevia) and in the plant Rubus chingii (Rosaceae).[1]
Steviol glycosides from Stevia rebaudiana have been reported to be between 30 and 320 times sweeter than sucrose,[2] although there is some disagreement in the technical literature about these numbers.[1][3] They are heat-stable, pH-stable, and do not ferment.[2]
Steviol glycosides do not induce a glycemic response when ingested, because humans cannot metabolize stevia.[4][5] The acceptable daily intake (ADI) for steviol glycosides, expressed as steviol equivalents, has been established to be 4 mg/kg body weight/day, and is based on no observed effects of a 100 fold higher dose in a rat study.[6]
Structure

These compounds are glycosides of steviol. Specifically, their molecules can be viewed as a steviol molecule, with its carboxyl hydrogen atom replaced by a glucose molecule to form an ester, and a hydroxyl hydrogen with combinations of glucose and rhamnose to form an acetal.
The steviol glycosides found in S. rebaudiana leaves, and their dry weight percentage, include:
- Stevioside (5–10%)
- Dulcoside A (0.5–1%)
- Rebaudioside A (2–4%)
- Rebaudioside B
- Rebaudioside C (1–2%)
- Rebaudioside D
- Rebaudioside E
- Rebaudioside F
- Rubusoside
- Steviolbioside
The last three are present only in minute quantities, and rebaudioside B has been claimed to be a byproduct of the isolation technique.[2] A commercial steviol glycoside mixture extracted from the plant was found to have about 80% stevioside, 8% rebaudioside A, and 0.6% rebaudioside C.[3]
The Chinese plant Rubus chingii produces rubusoside, a steviol glycoside not found in Stevia.[1] According to the EU Stevia Regulation of 13 July 2021, however, rubusoside is one of the eleven major glycoside components of Stevia, extracted from the leaves of the Stevia rebaudiana.[7]
Stevioside and rebaudioside A were first isolated in 1931 by French chemists, Bridel and Lavielle.[8] Both compounds have only glucose subgroups: stevioside has two linked glucose molecules at the hydroxyl site, whereas rebaudioside A has three, with the middle glucose of the triplet connected to the central steviol structure.
Early sensory tests led to claims that rebaudioside A was 150 to 320 times sweeter than sucrose, stevioside was 110 to 270 times sweeter, rebaudioside C 40 to 60 times sweeter, and dulcoside A 30 times sweeter.[2] However, a more recent evaluation found rebaudoside A to be about 240 times sweeter, and stevioside about 140 times.[1] Rebaudioside A also had the least bitterness and aftertaste.[2] The relative sweetness seems to vary with concentration: a mix of steviol glycosides in the natural proportions was found to be 150 times sweeter than sucrose when matching a 3% sucrose solution, but only 100 times sweeter when matching a 10% sucrose solution.[3]
Biosynthesis
In Stevia rebaudiana, the biosynthesis of the glucosides occurs only in green tissues. Steviol is first produced in the plastids and in the endoplasmic reticulum is glucosylated and glycosylated in the cytoplasm, catalyzed by UDP-glucosyltransferases. Rebaudioside A, in particular, is formed from stevioside.

Though there are several molecules that fall into the category of steviol glycoside, synthesis follows a similar route.[9] Synthesis of steviol glycoside begins with isoprene units created via the DXP or MEP pathway.[10][11] Two molecules derived from primary metabolism, Pyruvate and Glyceraldehyde 3-Phosphate, are the initial molecules for this pathway.
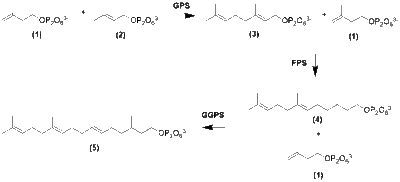
Upon forming IPP and DMAPP, the diterpene GGPP is formed by via head-to-tail addition by an Sn1 mechanism. Elongation begins when IPP and DMAPP form Geranyl Pyrophosphate (GPP). GPP elongates through the same Sn1 mechanism to create Farnesyl Pyrophosphate (FPP), and FPP elongates to form GGPP.
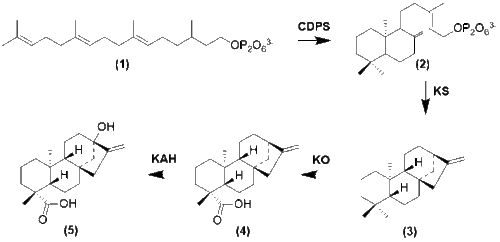
With the formation of GGPP cyclization occurs by enzymes copalyl diphosphate synthase (CDPS) and Kuarene Synthase (KS) to form -(-)Kuarene.[12] Several oxidation steps then occur to form steviol.
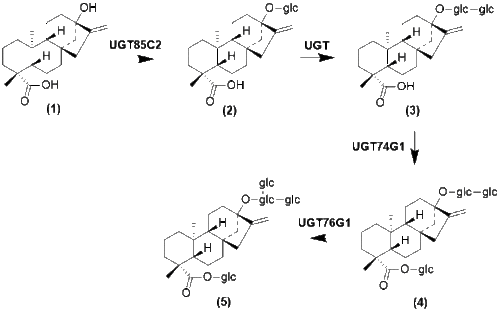
Steviol glycoside biosynthesis then follows several modifications from steviol that regioselectively select for sugar molecules to be placed.[13] Once these molecules are fully glycosylated, the glycosides are then stored in vacuoles.[1]
See also
References
- 1 2 3 4 5 Brandle, J. E.; Telmer, P. G. (2007). "Steviol glycoside biosynthesis" (PDF). Phytochemistry. 68 (14): 1855–1863. doi:10.1016/j.phytochem.2007.02.010. PMID 17397883. Archived from the original (PDF) on 2017-08-10.
- 1 2 3 4 5 Brandle, J. E.; Starratt, A. N.; Gijzen, M. (1998). "Stevia rebaudiana: Its agricultural, biological, and chemical properties". Canadian Journal of Plant Science. 78 (4): 527–536. doi:10.4141/P97-114.
- 1 2 3 H.M.A.B. Cardello; M.A.P.A. Da Silva; M.H. Damasio (1999). "Measurement of the relative sweetness of stevia extract, aspartame and cyclamate/saccharin blend as compared to sucrose at different concentrations". Plant Foods for Human Nutrition. 54 (2): 119–129. doi:10.1023/A:1008134420339. PMID 10646559. S2CID 38718610.
- ↑ Geuns, JM; Buyse, J; Vankeirsbilck, A; Temme, EH; Compernolle, F; Toppet, S (5 April 2006). "Identification of steviol glucuronide in human urine". Journal of Agricultural and Food Chemistry. 54 (7): 2794–8. doi:10.1021/jf052693e. PMID 16569078.
- ↑ Samuel P, Ayoob KT, Magnuson BA, Mathews R (2018). "Stevia Leaf to Stevia Sweetener: Exploring Its Science, Benefits, and Future Potential". Journal of Nutrition. 148 (7): 1186S–1205S. doi:10.1093/jn/nxy102. PMID 29982648.
- ↑ EFSA Panel on Food Additives and Nutrient Sources added to food (ANS) (2010). "Scientific Opinion on the safety of steviol glycosides for the proposed uses as a food additive". EFSA Journal. 8 (4): 1537. doi:10.2903/j.efsa.2010.1537.

- ↑ COMMISSION REGULATION (EU) 2021/1156 of 13 July 2021
- ↑ Bridel, M.; Lavielle, R. (1931). "Sur le principe sucré des feuilles de Kaâ-hê-é (stevia rebaundiana B)". Comptes rendus de l'Académie des sciences (Parts 192): 1123–1125.
- ↑ Huxtable, R.J., 2002. Pharmacology and toxicology of stevioside, rebaudioside A, and steviol. In: Kinghorn, A.D. (Ed.), Stevia: The Genus Stevia. Taylor and Francis, London and New York, pp.160–177.
- ↑ Lichtenhalter, H.K., 1999. The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu. Rev. Plant Physiol. PlantMol. Biol. 50, 47–65.
- ↑ Totté, N., Charon, L., Rohmer, M., Compernolle, F., Baboeuf, I., Geuns, J.M.C., 2000. Biosynthesis of the diterpenoid steviol, an ent-kaurene derivative from Stevia rebaudiana Bertoni, via the methylerythritol phosphate pathway Tetrahedron Letters 41, 6407–6410
- ↑ Richman, A.S., Gijzen, M., Starratt, A.N., Yang, Z., Brandle, J.E., 1999. Diterpene synthesis in Stevia rebaudiana: recruitment and up-regulationof key enzymes from the gibberellin biosynthetic pathway The Plant Journal 19, 411–421.
- ↑ Richman, A., Swanson, A., Humphrey, T., Chapman, R., McGarvey, B., Pocs, R., Brandle, J., 2005. Functional genomics uncovers threeglucosyltransferases involved in the synthesis of the major sweetglucosides of Stevia rebaudiana Plant J. 41, 56–67
External links
 Media related to Steviol glycosides at Wikimedia Commons
Media related to Steviol glycosides at Wikimedia Commons